Yellow fever (YF) is a viral haemorrhagic fever transmitted by infected Aedes mosquitoes. It is on the IDSR list of priority diseases for immediate reporting in Nigeria. Given the zoonotic nature of YF, NCDC has continued to adopt a ‘One Health’ approach to ensure Nigerians are protected against this disease. This is through collaboration with the Ministry of Agriculture and Rural Development, Ministry of Environment, World Health Organization (WHO) and other relevant development partners.
Although YF is a vaccine preventable disease, cases are still detected in Nigeria all year round. One of the priority areas for Nigeria’s YF response is strengthening diagnostic capacity for timely detection and prompt response to YF outbreaks. In the last five years, Nigeria has established a network of YF laboratories with six (6) laboratories across all six geopolitical zones in Nigeria. Alongside this, some of these laboratories are part of the Global Yellow Fever, Measles and Rubella Laboratory Network.
Annually, WHO conducts laboratory accreditation for quality assurance and ensures operations are in line with the recommended global best practices. This year, annual laboratory accreditation exercises are underway for Nigerian laboratories in the Global Yellow Fever, Measles and Rubella Laboratory Network. In Nigeria, a second laboratory within the National YF Network, Yusuf Dantsoho Memorial Hospital (YDMH), Kaduna has been fully accredited by WHO for yellow fever diagnosis in 2021. The NCDC is working closely with other laboratories to attain accreditation. The NCDC Central Public Health Laboratory (CPHL), Lagos also attained this full accreditation for the year.
With the need for improved yellow fever diagnostic capacity in Nigeria, NCDC in collaboration with WHO conducted an in-country training for focal persons within the network of YF laboratories from 8th - 12th March 2020. The objectives of the training were to:
1. Train laboratory staff involved in YF serological testing using the MAC-ELISA protocol
2. Train laboratory staff in the network on basic biosafety principles and practices
The training was conducted in line with the global network IgM Antibody Capture Enzyme-Linked Immunosorbent Assay (MAC-ELISA) protocol. The expected outcomes are a highly skilled laboratory workforce, improved data management and an adequately prepared system to fulfil the major criteria for WHO accreditation.
The NCDC remains committed to working with states, WHO and other relevant stakeholders in strengthening YF diagnostic capacity for the timely detection of case(s), prompt response and ultimately outbreak control, in line with the EYE strategy objectives.
Summary of Incidents
Notes
1. Information for this disease was retrieved from the Technical Working Group and Situation Reports
2. Case Fatality Rate (CFR) for this disease is reported for confirmed cases only
3. Information for this disease was retrieved from IDSR 002 data
4. CFR for this disease is reported for total cases i.e. suspected + confirmed
5. Information for sentinel influenza was retrieved from the laboratory

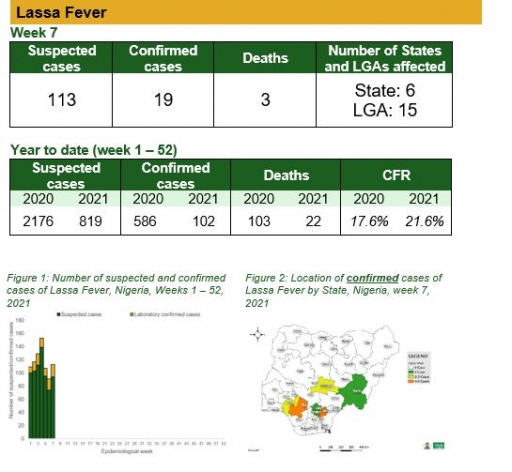
Lassa Fever
Key points
• There were 113 suspected cases, 19 were laboratory confirmed and three deaths were recorded from fifteen LGAs in six states
Actions
To date:
• Conducted 2021 Lassa fever high burden states preparedness/response engagement meeting
• Dissemination of reviewed case management and safe burial practices guidelines
• Implementation of Lassa fever Environmental response campaign in high burden states by Federal Ministry of Environment
Planned:
• Finalise Lassa fever five-year strategic plan
Cerebrospinal Meningitis (CSM)
Key points
• There were four suspected cases of Cerebrospinal Meningitis (CSM) reported from three LGAs in two states (Ebonyi – 3 & Oyo – 1). None were laboratory confirmed and no death was recorded
Actions
To date:
• National CSM TWG meets weekly to review reports from states and plan appropriately
• Enhanced surveillance in all states
Planned:
• Continue harmonisation of the national line list and SORMAS data
• Continue to ensure that states reporting cases send their line lists and collect CSM samples

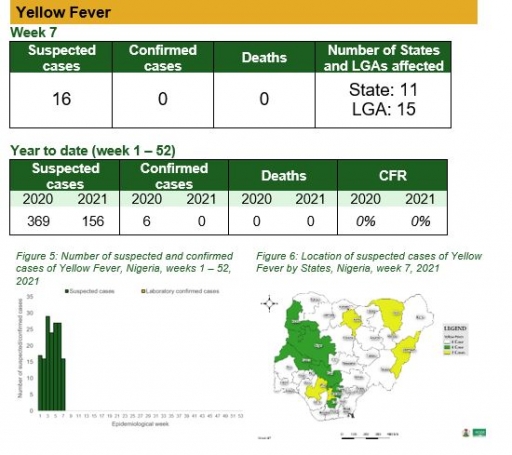
Yellow Fever
Key points
• There were 16 suspected cases of Yellow Fever (YF) reported from 15 LGAs in 11 states. None were laboratory confirmed and no death was recorded
Actions
To date:
• National YF multi-partner Technical Working Group (TWG) continues to coordinate activities across states.
• Daily monitoring and analysis of surveillance data across the country to guide response activities
• Ongoing training of focal persons within YF laboratories network on YF serological testing using MAC-ELISA protocol
Planned:
• Continue to support affected states across all pillars of response
• Continue harmonisation of surveillance and laboratory data ongoing
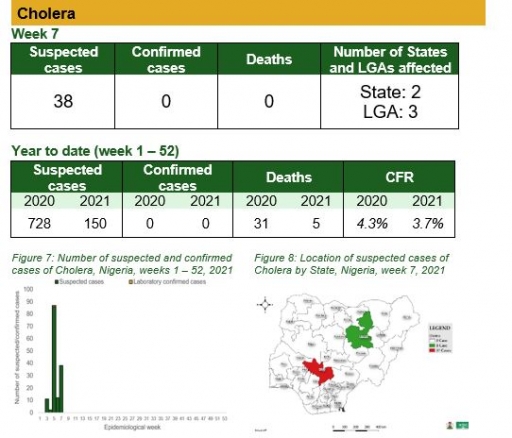
Cholera
Key points
• There were 38 suspected cases of Cholera reported from three LGAs in two states (Bauchi – 1 & Kogi – 37). None were laboratory confirmed and no death was recorded
Actions
To date
• National Cholera Multi-Sectoral Technical Working Group (TWG) is monitoring all states and supporting affected states
Planned:
• Continue follow up and monitoring of non-reporting states
• Continue harmonisation of the national line list and SORMAS data
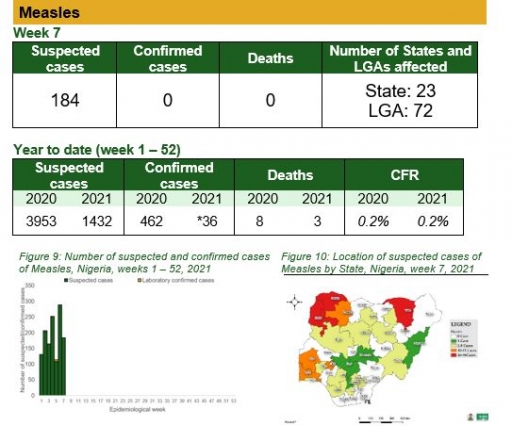
Measles
Key points
• There were 184 suspected cases of Measles reported from 72 LGAs in 23 states. None were laboratory confirmed and one death was recorded
Actions
To date
• National Measles TWG is closely monitoring measles surveillance data and providing feedback to relevant agencies and development partners
• Weekly surveillance and laboratory data harmonisation ongoing
Planned:
• Intensify follow up with states to update and transmit line list
• Continue monthly measles surveillance data review
Note:
• *Outbreak data added to routine data
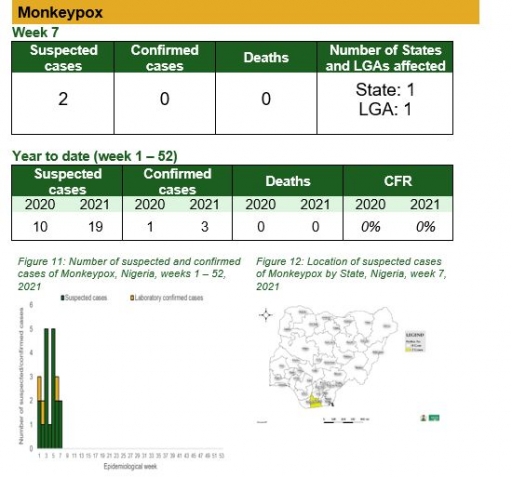
Monkeypox
Key points
• There were two suspected case of monkeypox reported from Gokana LGA in Rivers State. None were laboratory confirmed and no death was recorded
Actions
To date
• National Monkeypox Technical Working Group (TWG) is monitoring activities in all states
Planned:
• Enhance surveillance for Monkeypox in high burden states
• Continue harmonisation of the national line list and SORMAS data
Acute Flaccid Paralysis (AFP)

National Influenza Sentinel Surveillance

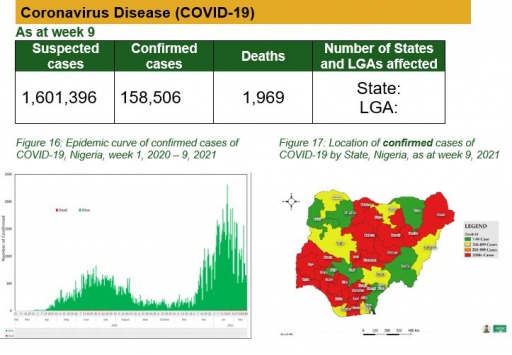
Coronavirus Disease (COVID-19)
Actions
To date:
• National COVID-19 multi-partner Emergency Operations Centre (EOC) continues to coordinate response activities across states
• Held virtual strategic coordination meeting with State Incident Managers, State Epidemiologists on improving coordination in the states
• Ongoing nationwide monthly distribution of medical and laboratory commodities to the State Ministries of Health (including the treatment and sample collection centres) and Teaching Hospitals across the country
• Ongoing roll-out of antigen-based Rapid Diagnostic Test in FCT
Planned:
• Deploy additional Rapid Response Teams to support states
• Finalise Local Government Area (LGA)/State transmission categorisation
• Support supervisory visits to private laboratories in FCT
Timeliness and Completeness of Reports
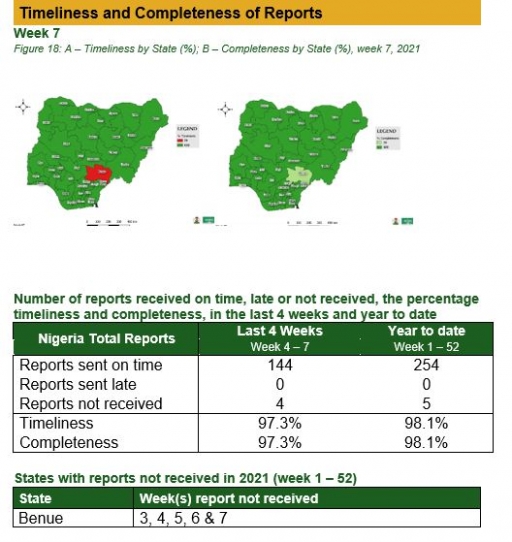
Timeliness and Completeness of Reports by State















 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 