This week’s editorial provides information regarding the preparatory efforts by the Nigeria Centre for Disease, relevant Ministries, Departments and Agencies (MDAs) and relevant development partners towards the mid-term Joint External Evaluation (JEE) of Nigeria’s International Health Regulations (IHR 2005) capacities. Our editorial for the Weekly Epidemiological Report for week 40 (see link) highlighted the introduction of the “National Action Plan for Health Security (NAPHS)†technical leads to the use of the JEE 2.0 tool.
Since the first Joint External Evaluation (JEE) of Nigeria’s International Health Regulations (IHR 2005) capacities in 2017, resources have been invested in bridging the identified gaps and implementing activities within the NAPHS. To answer the questions “Where we were (2017), Where we are (2019), and Where we intend to be (2022)?†the NCDC will hold its mid-term JEE review workshop from 18th -22nd November, 2019.
To ensure technical leads are better prepared for the mid-term JEE workshop process, NCDC in collaboration with relevant MDAs, and development partners on the 14th of November 2019 organised a multi-stakeholders workshop. The objectives were to:
The major outcomes from the workshop were the self f-assessment of current progress and challenges in implementation of NAPHS using JEE 2.0 tool as well as identification of immediate next steps to implement in 2020.
Between the 18th and 22nd of November 2019, the Government of Nigeria through its MDAs will present the results of the self-assessment to external evaluators, led by the World Health Organization (WHO).
As the national focal point for IHR implementation in Nigeria, NCDC remains committed to working closely with other MDAs, to strengthen the capacity for national health security. We remain grateful to our partners for supporting this process.
Summary of Incidents
Notes
1. Information for this disease was retrieved from the Technical Working Group and Situation Reports
2. Case Fatality Rate (CFR) for this disease is reported for confirmed cases only
3. Information for this disease was retrieved from IDSR 002 data
4. CFR for this disease is reported for total cases i.e. suspected + confirmed
5. Information for sentinel influenza was retrieved from the laboratory
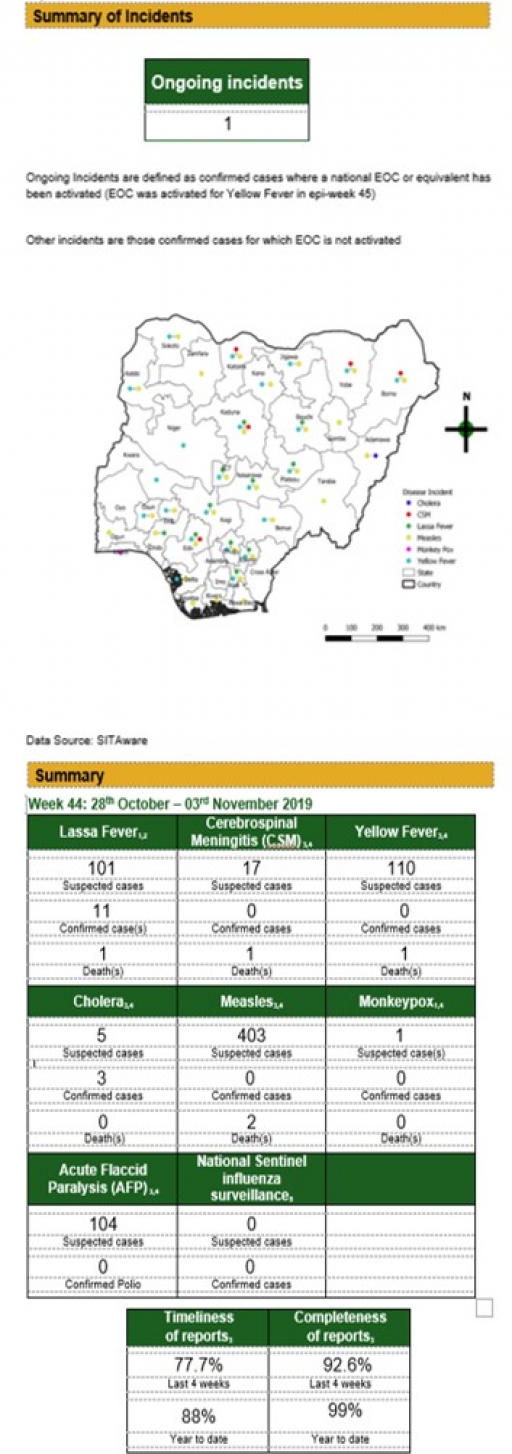
Lassa Fever
Key points
• There were 101 suspected cases of Lassa Fever (LF) reported from 40 LGAs in 11 states and FCT (Edo – 54, Ondo – 21, Ebonyi – 9, Bauchi – 6, Nasarawa – 1, Plateau – 3, FCT – 1, Kaduna – 2, Kogi – 1, Enugu – 1, Ogun – 1 & Abia – 1). There were 11 confirmed cases and one death was recorded
Actions
To date:
• The national Lassa Fever (LF) multi-partner, multi-sectoral Technical Working Group (TWG) continues to coordinate the response activities at all levels
• Harmonisation of LF laboratory, case management and surveillance data to SORMAS is ongoing
Planned:
• Finalise LF psychosocial guideline in November 2019
• Review LF case management and surveillance tools and SOPs in November 2019
• Implement rodent control measures in hotspot LGAs (Phase 2)
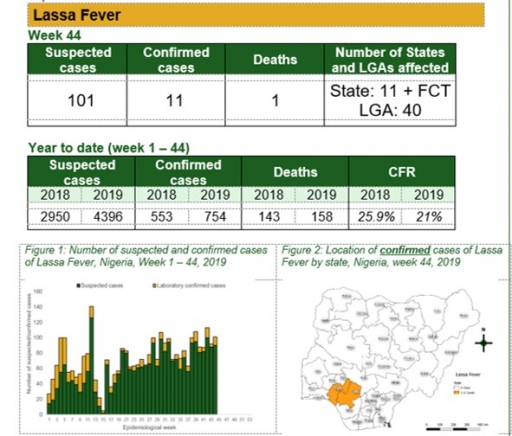
Cerebrospinal Meningitis (CSM)
Key points
There were 17 suspected cases of Cerebrospinal Meningitis (CSM) reported from nine LGAs in five states (Borno – 1, Edo – 2, Kaduna – 1, Katsina – 12, Yobe – 1). None was laboratory confirmed and one death was recorded
Actions
To date:
• The national CSM TWG meets weekly to review reports from states and plan appropriately
Planned:
• Continue harmonisation of the national line list and SORMAS data
• Collate CSM risk assessment, preparedness and response checklist for 2019/2020 from states to reflect 2018/2019 CSM response
• Continue to work closely with Katsina state’s team for further investigation of CSM cases and ensure proper sample collection
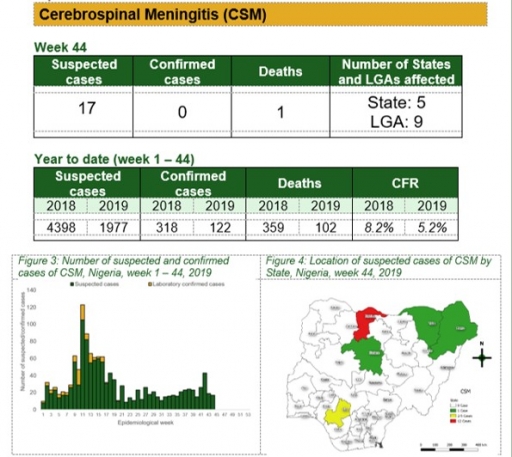
Yellow Fever
Key points
• There were 110 suspected cases of Yellow Fever (YF) reported from 56 LGAs in 22 states and FCT. None was laboratory confirmed and one death was recorded
Actions
To date:
• The national Yellow Fever (YF) Technical Working Group to continues to coordinate response activities
• Following up with the new states with confirmed cases (Taraba and Plateau states)
• Rapid Response Teams (RRT) have been deployed to Benue and Katsina states
Planned:
• Provide technical assistance to Bauchi state to conduct detailed investigation in Ningi LGA
• Follow up with NPHCDA on the pre-implementation plans for yellow fever preventive/reactive mass vaccination campaigns in the implementing LGAs/states
• Provide update from States to Risk Communication pillar for Action
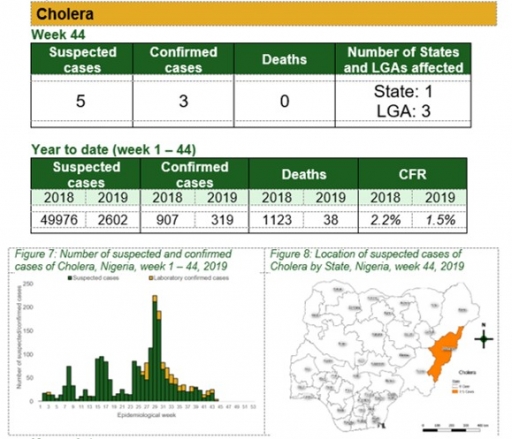
Cholera
Key points
• There were five suspected cases of cholera reported from three LGAs in Adamawa state. There were three laboratory confirmed cases and no death was recorded
Actions
To date
• The national Cholera multisectoral Technical Working Group (TWG) is monitoring all states and supporting already affected states
• Collected samples tested at the NCDC Central Public Health Laboratory Lagos
• Deploy a team from NCDC to carry out investigation of the ongoing outbreak in Lagos State
• Communication team working with relevant TWGs to develop flood advisories
Planned:
• Follow up with states with active outbreak and monitor non-reporting states
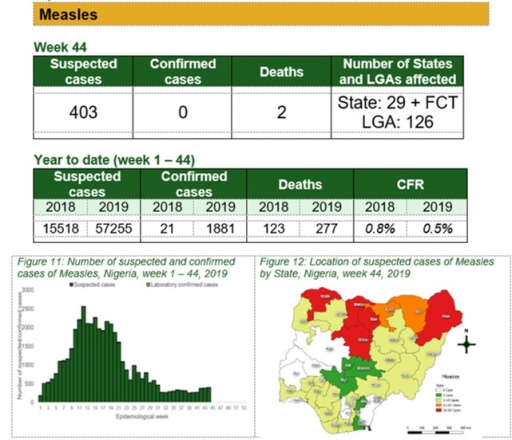
Measles
Key points
• There were 403 suspected cases of measles reported from 126 LGAs in 29 states and FCT. None was laboratory confirmed and two deaths was recorded
Actions
To date
• The national measles TWG is closely monitoring surveillance data and response activities across the country
Planned:
• Continue review of measles surveillance data across the country
• Follow up with Katsina surveillance team to obtain measles line list
• National TWG to be represented at the Measles Elimination Verification committee meeting scheduled for 11th to 13th November
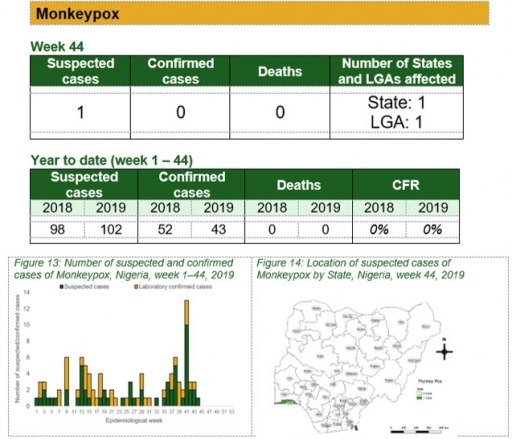
Monkeypox
Key points
• There was one suspected case of monkeypox reported from one LGA in Lagos state. The case was not confirmed and no death was recorded
Actions
• The national monkeypox TWG is monitoring activities in all states
• Surveillance has been enhanced in all affected states
Planned
• Regional monkeypox surveillance training in South East, South West and North Central in November 2019
• Capture monkeypox data into SITAWARE for real time reporting
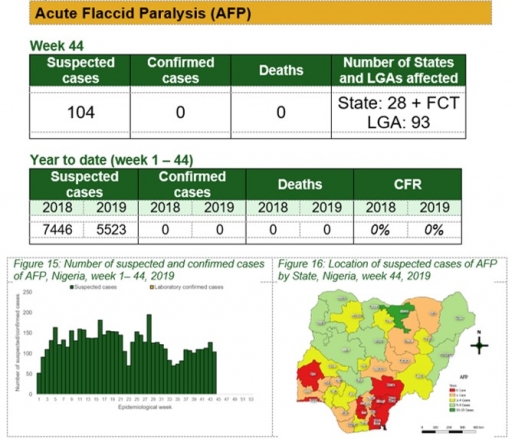
Acute Flaccid Paralysis (AFP)
Key points
• There were 104 suspected cases of AFP reported from 93 LGAs in 28 states and FCT. None was laboratory confirmed and no death was recorded
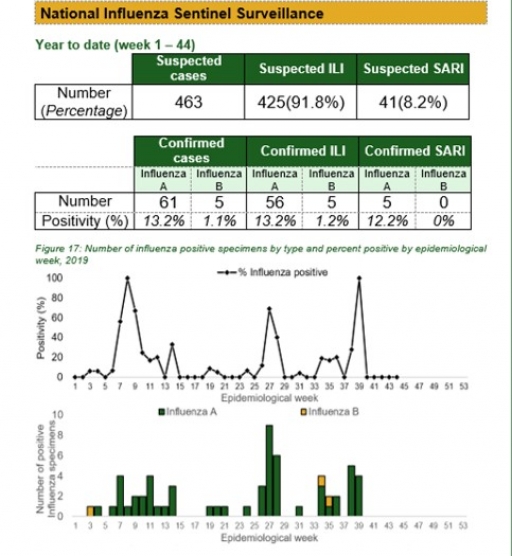
National Influenza Sentinel Surveillance
Key points
There were 67 processed samples positive for influenza, with 61 for influenza A, 5 for influenza B and 1 for influenza A & B
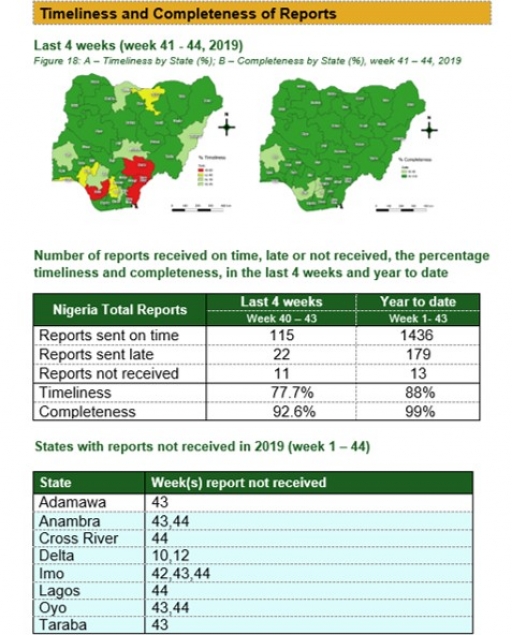
Timeliness and Completeness of Reports
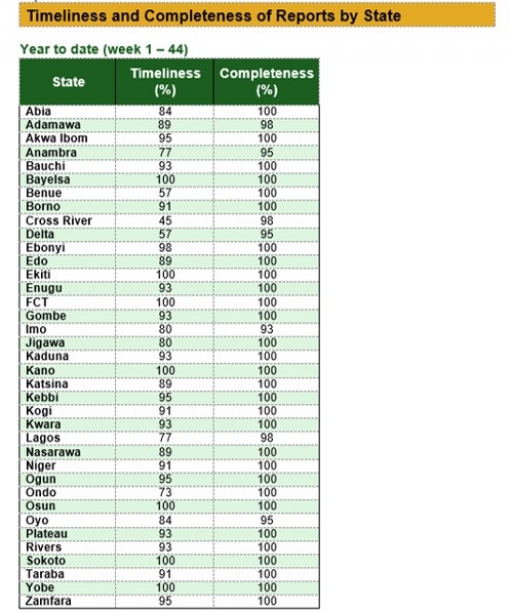
Timeliness and Completeness of Reports by State













 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 