In the 22nd week of the ongoing Cerebrospinal Meningitis (CSM) outbreak, four (4) additional Local Government Areas (LGAs) were affected, bringing the total of affected LGAs to 226. So far, a total of 14,005 suspected cases have been identified from 23 States and the Federal Capital Territory. Out of 901 samples sent for laboratory testing, 423 (46.9%) were confirmed positive for Neisseria meningitidis. 73% (309) of tested samples showed the causative organism to be Neisseria meningitidis serogroup C. The number of deaths recorded so far is 1,114 giving a case fatality rate (CFR) of 8%.
The increase in the number of suspected cases may be linked to an increased knowledge of the case definition for CSM. However, this does not translate to better outcome for the cases, as seen with the number of deaths recorded so far. Case management of CSM, like other disease conditions, follows a sequence of case detection, laboratory confirmation, treatment using the preferred drug of choice and supportive care.
At the onset of the outbreak, a large number of identified cases did not have samples taken for laboratory testing and were managed empirically. This may have been a contributory factor to the high number of deaths recorded. However, the Rapid Response Teams deployed to the most affected States supported sample collection and therefore an increase in laboratory confirmed cases.
Proper management of cases following approved protocols and guidelines is critical to the effective case management of any disease condition. States have the responsibility of ensuring that approved guidelines and protocols for case management are available in all health facilities/treatment centres. Furthermore, given that CSM is a seasonal event of public health concern, preparedness for the outbreak should entail community sensitization on the disease to promote early presentation and use of health facilities for immediate and proper institution of treatment/management
In the course of the outbreak, the Nigeria Centre for Disease Control (NCDC) alongside the National Primary Health Care Development Agency (NPHCDA) and other partner agencies have provided on-site support for case management in the worst affected states. A total of 10 teams (5 in each State) have been deployed to Zamfara and Sokoto States to improve sample collection rates, proper institution of treatment and other supportive care of confirmed cases. This action has improved the outcomes of cases (decreased Case Fatality Rate from above 8.0% to less than 8%) with attendant transfer of knowledge on case management protocols to health workers in both States. Also, Lumbar puncture rate increased from less than 50% to 71% in Zamfara State while it increased from less than 10% to 33% in Sokoto State. States are enjoined to continue to improve on best practices identified and ensure that high standards of case management are employed at all times.
In the reporting week:
o There were 308 new cases of Acute Flaccid Paralysis (AFP) reported. None was confirmed as Polio. The last reported case of Polio in Nigeria was in August 2016. Active case search for AFP is being intensified as Nigeria has assiduously reinvigorated its efforts at eradicating Polio.
o Five suspected cases of Cholera were reported from two LGAs in two States and no death was recorded.
o There were 343 suspected cases of Cerebrospinal Meningitis (CSM) reported from 58 LGAs in 15 States. Of these, two were laboratory confirmed and 13 deaths were recorded. Surveillance for CSM is ongoing and intensified in the States.
o There were 406 suspected cases of Measles reported from 31 States including the FCT. Three laboratory confirmed cases were recorded with no deaths.
In the reporting week, Adamawa State failed to report. Timeliness of reporting increased from 80% in the previous week to 81% in the current week while completeness increased from 99.0% in the previous week to 100% in the current week. It is very important for all States to ensure timely and complete reporting at all times.
1. Lassa fever
Please note that the data reflects the routine reports i.e. all suspected cases including the laboratory positive and negative cases
1.1. Two suspected cases of Lassa fever were reported from two LGAs (two States) in week 19, 2017 compared with zero during the same period in 2016.
1.2. Laboratory results of the two suspected cases were negative (Cross River – 1 and Nassarawa – 1).
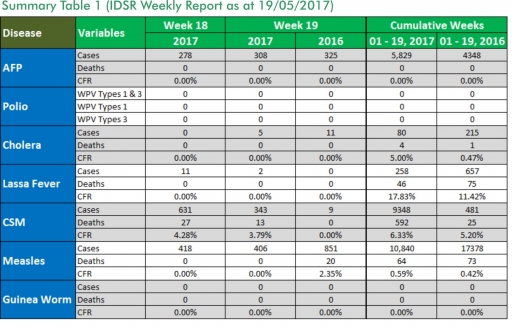
1.3. Between weeks 1 and 19 (2017), 258 suspected Lassa fever cases with 58 laboratory confirmed cases and 46 deaths (CFR, 17.83%) from 51 LGAs (20 States) were reported compared with 657 suspected cases with 63 laboratory confirmed cases and 75 deaths (CFR, 11.42%) from 122 LGAs (27 States) during the same period in 2016 (Figure 1).
1.4. Between weeks 1 and 52 2016, 921 suspected Lassa fever cases with 109 laboratory confirmed cases and 119 deaths (CFR, 12.92%) from 144 LGAs (28 States and FCT) were reported compared with 430 suspected cases with 25 laboratory confirmed cases and 40 deaths (CFR, 9.30%) from 37 LGAs (14 States and FCT) during the same period in 2015 (Figure 2).
1.5. Investigation and active case search ongoing in affected States with coordination of response activities by the NCDC with support from partners.
1.5.1. National Lassa Fever Working Group meeting and weekly National Surveillance and Outbreak Response meeting on-going at NCDC to keep abreast of the current Lassa fever situation in the country.
1.5.2. Response materials for VHFs prepositioned across the country by NCDC at the beginning of the dry season
1.5.3. New VHF guidelines have been developed by the NCDC (Interim National Viral Haemorrhagic Fevers Preparedness guidelines and Standard Operating Procedures for Lassa fever management)
1.5.4. Ongoing reclassification of reported Lassa fever cases
1.5.5. Ongoing review of the variables for case-based surveillance for VHF
1.5.6. VHF case-based forms completed by affected States are being entered into the new VHF management system. This system allows for the creation of a VHF database for the country.
1.5.7. Confirmed cases are being treated at identified treatment/isolation centres across the States with Ribavirin and necessary supportive management also instituted
1.5.8. Onsite support was earlier provided to Ogun, Nasarawa, Taraba, Ondo and Borno States by the NCDC and partners
1.5.9. Offsite support provided by NCDC/partners in all affected States
1.5.10. States are enjoined to intensify surveillance
2. MEASLES
2.1. In the reporting week, 406 suspected cases of Measles with three laboratory confirmed cases were reported from 31 States and FCT compared with 851 suspected measles cases and 20 deaths (CFR, 2.35%) from 29 States during the same period in 2016.
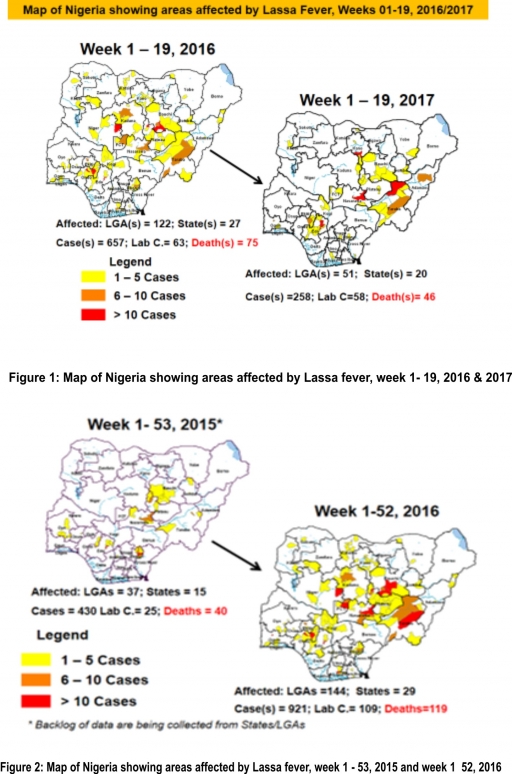
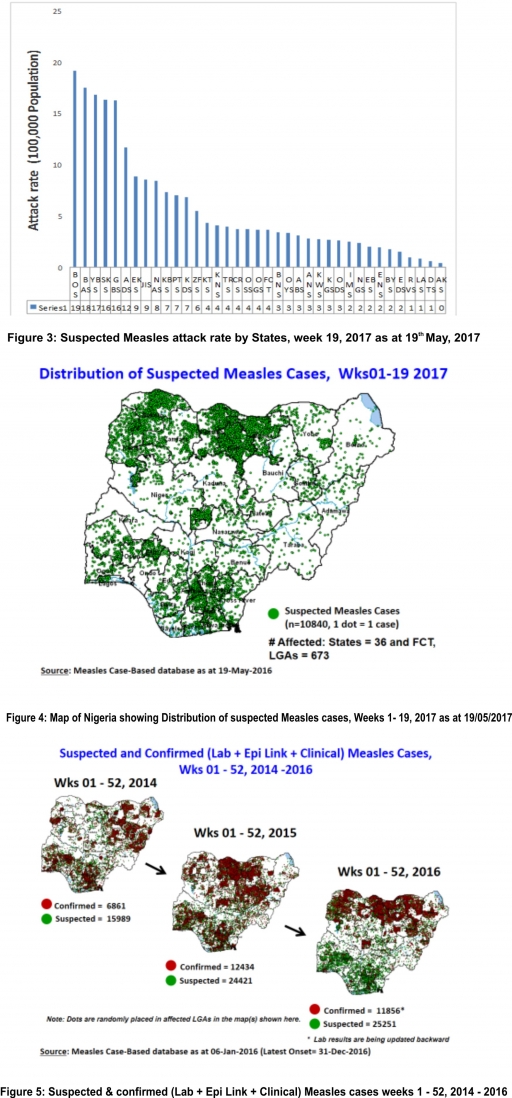
2.2. So far, 10,840 suspected Measles cases with 69 laboratory confirmed cases and 64 deaths (CFR, 0. 59%) have been reported in 2017 from 36 States and FCT (Figure 4) compared with 17,378 suspected cases and 73 deaths (CFR, 0.42%) from 36 States and FCT during the same period in 2016.
2.3. In 2016 (week 1 -52), 25,251 suspected Measles cases with 102 deaths (CFR, 0.40%) were reported from 36 States and FCT compared with 24,421 suspected cases with 127 deaths (CFR, 0.52%) during the same period in 2015 (Figure 5)
2.4. Response measures include immunization for all vaccine-preventable diseases in some selected/affected wards/LGAs during SIAs, as well as case management.
2.5. Scheduled Measles campaigns in the North East were conducted from 12th – 17th January, 2017 in Adamawa, Borno and Yobe States (Phase I) and Phase II from 21st – 25th January, 2017 in Borno State and 4th – 8th February, 2017 in Yobe State
3. POLIOMYELITIS
3.1. As at May 12th 2017, no new case of WPV was recorded
3.2. Three new cVDPV2, environmental derived and Polio compatible cases identified
3.2.1. In the reporting week, 308 cases of AFP were reported from 225 LGAs in 33 States and FCT
3.2.2. AFP Surveillance has been enhanced and outbreak response is on-going in Borno and other high risk States
3.2.3. The 1st round of SIPDs in 2017 was conducted from 28th – 31st January 2017 in the 18 high risk States. This was carried out using mOPV2 (2nd mOPV2 OBR). The schedule for other SIAs is as described in Table 2
3.2.4. The 2nd round of SIPDs completed (25th-28th February, 2017) in 14 high risk States using bOPV.
3.2.5. The 1st and 2nd rounds of NIPDs completed (from 25th – 28th March, 2017 and 22nd – 25th April, 2017) nationwide respectively.
3.2.6. Between weeks 1 and 52 in 2016, four WPVs were isolated from Borno State compared to no WPV isolated during the same period in 2015.
3.3. No circulating Vaccine Derived Polio Virus type 2 (cVDPV2) was isolated in week 1 - 52, in both 2016 and 2015.
3.4. Between weeks 1 and 52, 2016 two (2) cVDPV2 were isolated in two LGAs (two States) while one (1) cVDPV2 was isolated from Kwali, FCT during the same period in 2015.
3.5. Six confirmed WPVs were isolated in 2014.
3.6. The SIAs were strengthened with the following events:
3.6.1. Immunization for all vaccine-preventable diseases in some selected wards/LGAs.
3.6.2. Use of health camp facilities.
3.6.3. Field supportive supervision and monitoring.
3.6.4. Improved Enhanced Independent Monitoring (EIM) and Lots Quality Assessments (LQAs) in all Polio high risk States.
3.6.5. High level of accountability framework
4. CHOLERA
4.1. Five suspected cases of Cholera were reported from two LGAs (two States) in week 19 compared with 11 suspected cases from Kiru LGA (Kano State) at the same period in 2016.
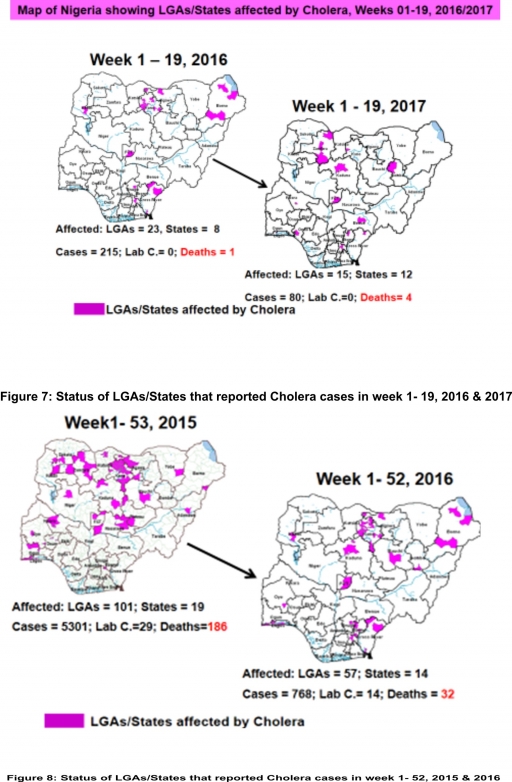
4.2. Between weeks 1 and 19 (2017), 80 suspected Cholera cases and four deaths (CFR, 5.0%) from 15 LGAs (12 States) were reported compared with 215 suspected cases and one death (CFR, 0.47%) from 23 LGAs (eight States) during the same period in 2016 (Figure 7).
4.3. Between weeks 1 and 52 (2016), 768 suspected Cholera cases with 14 laboratory confirmed cases and 32 deaths (CFR, 4.17%) from 57 LGAs (14 States) were reported compared with 5,301 cases with 29 laboratory confirmed cases and 186 deaths (CFR, 3.51%) from 101 LGAs (18 States and FCT) during the same period in 2015 (Figure 8).
4.4. States are enjoined to intensify surveillance.
5. CEREBROSPINAL MENINGITIS (CSM)
5.1. In the reporting week 19, 343 suspected Cerebrospinal Meningitis (CSM) cases with two Lab confirmed and 13 deaths (CFR, 3.79%) were reported from 58 LGAs (15 States) compared with nine suspected cases from six LGAs (six States) during the same period in 2016.
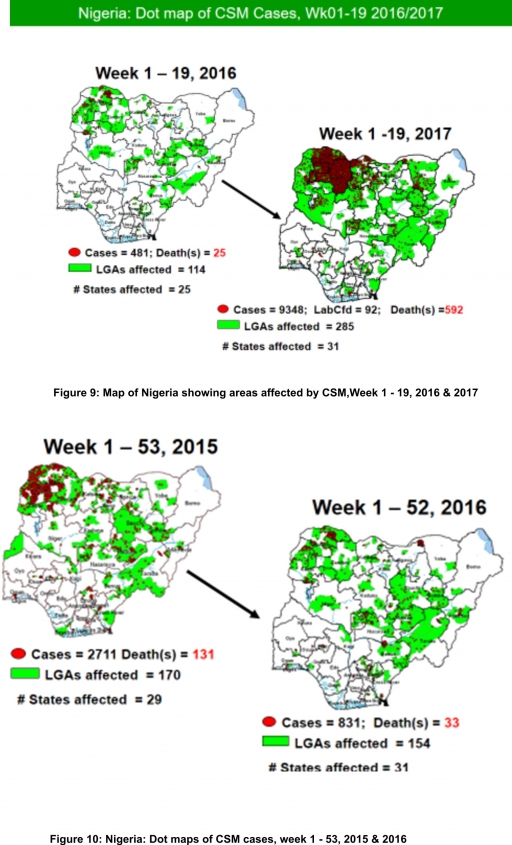
5.2. Between weeks 1 and 19 (2017), 9348 suspected CSM cases with 92 laboratory confirmed cases and 592 deaths (CFR, 6.33%) were recorded from 285 LGAs (31 States) compared with 481 suspected cases and 25 deaths (CFR, 5.20%) from 114 LGAs (25 States) during the same period in 2016 (Figure 9).
5.3. Between weeks 1 and 52, 2016, 831 suspected CSM cases with 43 laboratory confirmed cases and 33 deaths (CFR, 3.97%) were recorded from 154 LGAs (30 States and FCT) compared with 2,711 suspected cases and 131 deaths (CFR, 4.83%) from 170 LGAs (28 States and FCT) during the same period in 2015 (Figure 10)
5.4. Timeliness/completeness of CSM case-reporting from States to the National Level (2017 versus 2016): on average, 79.6% of the 26 endemic States sent CSM reports in a timely manner while 95.3% were complete in week 1 – 19, 2017 as against 82.4% timeliness and 96.6% completeness recorded within the same period in 2016
5.5. CSM preparedness checklist sent to 36 States and FCT ahead of 2017 meningitis season
5.6. Confirmed cases are being treated at identified treatment centres in affected States (Zamfara, Sokoto, Katsina, Kebbi, Niger, Kano, Yobe and Jigawa) and necessary supportive management also instituted
5.7. CSM National Emergency Operations Centre constituted at the Nigeria Centre for Disease Control
5.8. Onsite support was earlier provided to Zamfara State and still ongoing.
5.9. Ongoing onsite support to Sokoto, Katsina, Kebbi, Kano and Niger States by NCDC and partners
5.10. Intensive Surveillance is on-going in high risk States.
5.11. Reactive vaccination completed in Zamfara State for people aged one to 29 years using polysaccharide meningococcal A & C vaccine.
5.12. Reactive vaccination completed in two wards (Gada and Kaffe) in Gada LGA in Sokoto State using polysaccharide meningococcal A & C vaccine for people aged two to 29 years.
5.13. Reactive vaccination completed in nine LGAs in Sokoto State using monosaccharide meningococcal conjugate C vaccine for aged one to 20 years.
5.14. Reactive vaccination campaign ongoing in Yobe State and the second phase of the campaign in Zamfara State is also ongoing.
5.15. Training and deployment of first batch of medical teams to support case management in Sokoto and Zamfara States from Friday 5th May, 2017 ongoing.
6. GUINEA WORM DISEASE
6.1. In the reporting week, no rumour report of Guinea Worm disease was received from any State.
6.2. Nigeria has celebrated eight consecutive years of zero reporting of Guinea worm disease in the country. The Country has been officially certified free of Dracunculiasis transmission by the International Commission for the Certification of Dracunculiasis Eradication (ICCDE).
(For further information, contact NIGEP NC/Director: Mrs. I, Anagbogu: +2348034085607, [email protected])
FOR MORE INFORMATION CONTACT
Surveillance Unit:
Nigeria Centre for Disease Control
801 Ebitu Ukiwe Street, Jabi, Abuja, Nigeria.
[email protected]
www.ncdc.gov.ng/reports
0800-970000-10










 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 