On the 12th of September 2017, a confirmed case of Yellow fever was reported in Ifelodun Local Government Area of Kwara state, Nigeria. This is the first confirmed case of the disease reported in the country in over ten years. Pertinent questions to guide a detailed public health response revolve around early case identification, sensitivity of the surveillance system particularly at points of entry, immunisation coverage for the disease and possible effects of climatic changes on established disease epidemiology.
Daily movement of people and goods across borders for travel and trade puts a country at risk of spread of any infectious disease. With the recent confirmation of Yellow fever in Kwara, it is very important for surveillance activities at various points of entry and health facilities in the country to be heightened. At the ports, it is important that every traveler in and out of the country has a valid yellow fever immunisation card. It is also important for health workers and disease surveillance officers to promptly notify relevant authorities of suspected cases, by the fastest means necessary.
The Integrated Disease Surveillance and Response (IDSR) technical guidelines provides the standard case definition for yellow fever which should be used in all health care facilities across Nigeria. A summary of the case definition of Yellow fever is seen in the table below:
Suspected case Any person with acute onset of fever, with jaundice appearing within 14 days of onset of the first symptoms.
o Epidemiological link to a confirmed case or an outbreak
Confirmed Case 1. A probable case
o Detection of Yellow Fever(YF)-specific* Immunoglobulin M(IgM)
o Detection of four-fold increase in YF IgM and/or Immunoglobulin G(IgG) antibody titres between acute and
o Detection of YFV-specific* neutralizing antibodies
*YF-specific means that antibody tests (such as IgM or neutralizing antibody) for other prevalent flavivirus are
negative. This testing should include at least IgM for Dengue and West Nile and may include other flavivirus d
epending on local epidemiology.
2. One of the following:
o Detection of YF virus genome in blood or other organs by PCR
o Detection of yellow fever antigen in blood, liver or other organs by immunoassays
3. Isolation of the yellow fever virus
Community and healthcare worker sensitization are important strategies that should be employed by States in strengthening the surveillance system. This will ensure that health workers can identify suspected cases, and report same to the Local Government Disease Surveillance and Notification Officer (DSNO) or State Epidemiologist. This will also help to address the issue of stigmatization of cases and health facilities that manage cases.
Currently, the Kwara State Ministry of Health is leading the response to the confirmed case of Yellow fever, supported by the Nigeria Centre for Disease Control (NCDC), National Primary Health Care Development Agency (NPHCDA), National Arbovirus and Vectors Research Centre (NAVRC), World Health Organisation (WHO) Country Office and other partner agencies.
The combined Team is carrying out a detailed investigation and risk analysis with plans for a reactive vaccination campaign in the affected LGA and surrounding communities. An Incident Management System has also been constituted at the national level by the NCDC, to ensure rapid and coordinated decision-making.
A letter of alert has been issued to all States of the country. States are enjoined to commence awareness campaigns and community mobilization activities for yellow fever, emphasizing the importance of vaccination against the disease, and proper sanitation to prevent breeding of the Aedes mosquito. Early reporting of suspected cases of Yellow fever to the next level of authority is very important.
1. World Health Organization and Centers for Disease Control and Prevention (2013). Technical Guidelines for Integrated Disease Surveillance and Response in the African Region, Brazzaville, Republic of Congo and Atlanta, USA.
In the reporting week ending on the 10th of September, 2017:
o There were 206 new cases of Acute Flaccid Paralysis (AFP) reported. None was confirmed as Polio. The last reported case of Polio in Nigeria was in August 2016. Active case search for AFP is being intensified as Nigeria has assiduously reinvigorated its efforts at eradicating Polio.
o 708 suspected cases of Cholera were reported from ten LGAs (six States; Bauchi – 3, Borno – 593, Kaduna – 28, Kano – 4 and Oyo - 8). Four were laboratory confirmed and 18 deaths were recorded.
o 21 suspected cases of Lassa fever with three Laboratory confirmed and one deaths were reported from seven LGAs in six States (Edo – 4, FCT – 2, Gombe – 1, Kogi – 1, Ogun -1 & Ondo – 12).
o There were nine suspected cases of Cerebrospinal Meningitis (CSM) reported from seven LGAs in six States (Adamawa – 1, Benue – 1, Enugu – 1, Katsina – 4, Kogi – 1 and Niger - 1). Of these, none was laboratory confirmed and no death was recorded. Ongoing surveillance for CSM has been intensified in all the 26 States in the Nigeria meningitis belt.
o There were 206 suspected cases of Measles reported from 31 States. None was laboratory confirmed and no death was recorded.
In the reporting week, Abia State failed to send in their report. Timeliness of reporting remains 84% in both previous and current weeks (Week 35 and 36) while completeness remains at 100%. It is very important for all States to ensure timely and complete reporting at all times, especially during an outbreak.
1. Lassa fever
Please note that the data reflects the routine reports i.e. all suspected cases including the laboratory positive and negative cases
1.1. 21 suspected cases of Lassa fever with three Laboratory confirmed and one death (CFR, 4.76%) were reported from seven LGAs (six States; Edo – 4, FCT – 2, Gombe – 1, Kogi – 1, Ogun -1 and Ondo – 12) in week 36, 2017 compared with seven suspected cases and one deaths (CFR, 14.3%) reported from six LGAs (four States) at the same period in 2016.
1.2. Laboratory results of the 21 suspected cases were three positives for Lassa fever (Ondo -3) while 18 were negative for Lassa fever and other VHFs (Edo – 4, FCT- 2, Gombe – 1, Kogi – 1, Ogun-1 and Ondo - 9).
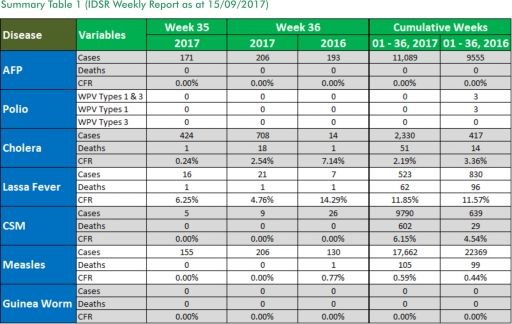
1.3. Between weeks 1 and 36 (2017), 523 suspected Lassa fever cases with 114 laboratory confirmed cases and 62 deaths (CFR, 11.85%) from 80 LGAs (26 States) were reported compared with 830 suspected cases with 80 laboratory confirmed cases and 96 deaths (CFR, 11.57%) from 135 LGAs (28 States) during the same period in 2016 (Figure 1).
1.4. Between weeks 1 and 52 2016, 921 suspected Lassa fever cases with 109 laboratory confirmed cases and 119 deaths (CFR, 12.92%) from 144 LGAs (28 States and FCT) were reported compared with 430 suspected cases with 25 laboratory confirmed cases and 40 deaths (CFR, 9.30%) from 37 LGAs (14 States and FCT) during the same period in 2015 (Figure 2).
1.5. Investigation and active case search ongoing in affected States with coordination of response activities by the NCDC with support from partners.
1.5.1. National Lassa Fever Working Group meeting and weekly National Surveillance and Outbreak Response meeting on-going at NCDC to keep abreast of the current Lassa fever situation in the country.
1.5.2. Response materials for VHFs provided to support States
1.5.3. New VHF guidelines have been developed by the NCDC (National Viral Haemorrhagic Fevers Preparedness guidelines, Infection Prevention and Control of VHF and Standard Operating Procedures for Lassa fever management) and are available on the NCDC website.
1.5.4. National Lassa fever outbreak review meeting carried out with all affected States and partners
1.5.5. Ongoing reclassification of reported Lassa fever cases
1.5.6. Ongoing review of the variables for case-based surveillance for VHF
1.5.7. VHF case-based forms completed by affected States are being entered into the new VHF management system. This system allows for the creation of a VHF database for the country.
1.5.8. Confirmed cases are being treated at identified treatment/isolation centres across the States with Ribavirin and necessary supportive management also instituted
1.5.9. Onsite support was earlier provided to Ogun, Nasarawa, Taraba, Ondo and Borno States by the NCDC and partners
1.5.10. Offsite support provided by NCDC/partners in all affected States
1.5.11. NCDC and partners are providing onsite support in Ondo and Plateau State
1.5.12. States are enjoined to intensify surveillance and promote Infection, Prevention and Control (IPC) measures in health facilities.
2. MEASLES
2.1. In the reporting week, 206 suspected cases of Measles were reported from 31 States compared with 130 suspected measles cases and 1 death (CFR, 0.77%) reported from 24 States during the same period in 2016.
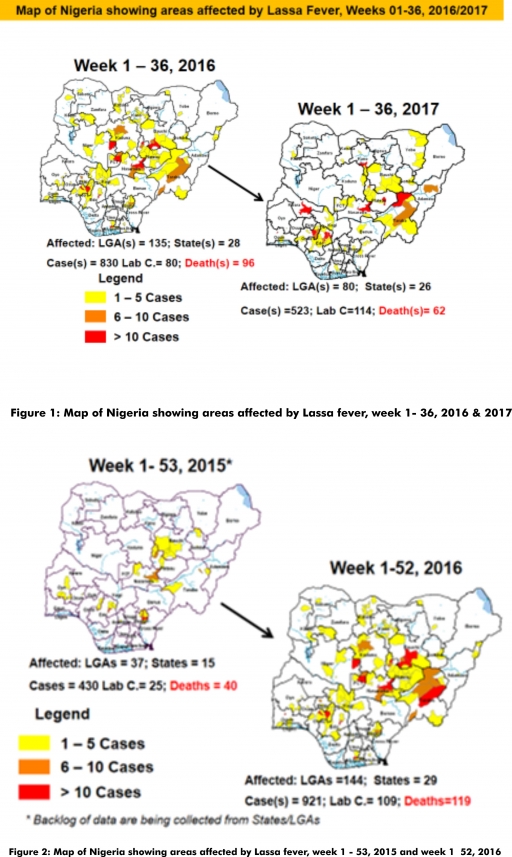
2.2. So far, 17,552 suspected Measles cases with 108 laboratory confirmed cases and 105 deaths (CFR, 0. 60%) have been reported in 2017 from 36 States and FCT (Figure 4) compared with 22,369 suspected cases and 99 deaths (CFR, 0.44%) from 36 States and FCT during the same period in 2016.
2.3. In 2016 (week 1 -52), 25,251 suspected Measles cases with 102 deaths (CFR, 0.40%) were reported from 36 States and FCT compared with 24,421 suspected cases with 127 deaths (CFR, 0.52%) during the same period in 2015 (Figure 5)
2.4. Response measures include immunization for all vaccine-preventable diseases in some selected/affected wards/LGAs during SIAs, as well as case management.
2.5. Scheduled Measles campaigns in the North East were conducted from 12th – 17th January 2017 in Adamawa, Borno and Yobe States (Phase I) and Phase II from 21st – 25th January 2017 in Borno State and 4th – 8th February 2017 in Yobe State
2.6. Measles Surveillance Evaluation and Establishment of the burden of Congenital Rubella Syndrome (CRS) in 12 selected States in the six geopolitical zones from the 17th -21st July 2017 conducted
2.6.1 Debrief meeting to review results and next steps from Measles evaluation conducted, held on the 15th of September 2017
2.7. Harmonization of measles surveillance data with laboratory-confirmed cases
3. POLIOMYELITIS
3.1. As at September 3rd 2017, no new case of WPV was recorded
3.2. Three new cVDPV2, environmental derived and Polio compatible cases identified
3.2.1. In the reporting week, 206 cases of AFP were reported from 165 LGAs in 31 States and FCT
3.2.2. AFP Surveillance has been enhanced and outbreak response is on-going in Borno and other high risk States
3.2.3. The 1st round of SIPDs in 2017 was conducted from 28th – 31st January 2017 in the 18 high risk States. This was carried out using mOPV2 (2nd mOPV2 OBR). The schedule for other SIAs is as described in Table 2
3.2.4. The 2nd and 3rd round of SIPDs completed (25th-28th February and 8th – 11th July, 2017) in 14 & 18 high risk States using bOPV respectively.
3.2.5. The 1st and 2nd rounds of NIPDs completed (from 25th – 28th March, 2017 and 22nd – 25th April, 2017) nationwide respectively.
3.2.6. Between weeks 1 and 52 in 2016, four WPVs were isolated from Borno State compared to no WPV isolated during the same period in 2015.
3.3. No circulating Vaccine Derived Polio Virus type 2 (cVDPV2) was isolated in week 1 - 52, in both 2016 and 2015.
3.4. Between weeks 1 and 52, 2016 two (2) cVDPV2 were isolated in two LGAs (two States) while one (1) cVDPV2 was isolated from Kwali, FCT during the same period in 2015.
3.5. Six confirmed WPVs were isolated in 2014.
3.6. The SIAs were strengthened with the following events:
3.6.1. Immunisation for all vaccine-preventable diseases in some selected wards/LGAs.
3.6.2. Use of health camp facilities.
3.6.3. Field supportive supervision and monitoring.
3.6.4. Improved Enhanced Independent Monitoring (EIM) and Lots Quality Assessments (LQAs) in all Polio high-risk States.
3.6.5. High level of accountability framework
4. CHOLERA
4.1. 708 suspected cases of Cholera with four Laboratory confirmed and 18 deaths (CFR, 2.54%) were reported from ten LGAs (six States; Bauchi – 3, Borno – 593, Kaduna - 28 Kano – 4 and Oyo - 8) in week 36 compared with 14 suspected cases and one death (CFR, 7.14%) reported from Oshodi/Isolo LGA in Lagos State during the same period in 2016.
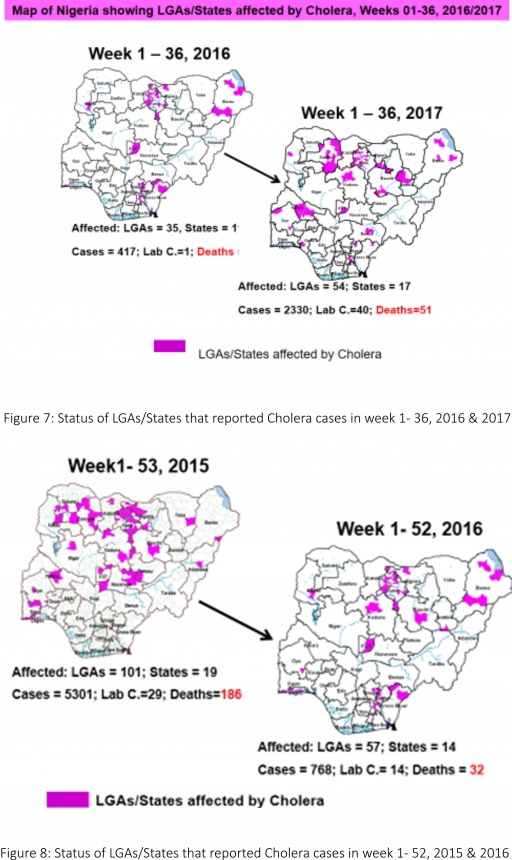
4.2. Between weeks 1 and 36 (2017), 2330 suspected Cholera cases with 40 laboratory confirmed and 51 deaths (CFR, 2.19%) from 55 LGAs (17 States) were reported compared with 417 suspected cases and 14 deaths (CFR, 3.36%) from 35 LGAs (11 States) during the same period in 2016 (Figure 7).
4.3. Between weeks 1 and 52 (2016), 768 suspected Cholera cases with 14 laboratory confirmed cases and 32 deaths (CFR, 4.17%) from 57 LGAs (14 States) were reported compared with 5,301 cases with 29 laboratory confirmed cases and 186 deaths (CFR, 3.51%) from 101 LGAs (18 States and FCT) during the same period in 2015 (Figure 8).
4.4. Cholera preparedness workshop held from 31st May – 1st June, 2017 in Abuja to
develop Cholera preparedness plan as the season set in.
4.5. NCDC/partners provided onsite support in Kwara, Zamfara and Kebbi States.
4.6 NCDC/partners are providing onsite support in Borno State.
4.7. Cholera Preparedness Checklist sent to all States to assess their level of preparedness with recommendations for prevention of and response to an outbreak.
4.8. RDT procured by NCDC and WHO currently being prepositioned in affected States
4.9. States are enjoined to intensify surveillance, implement WASH activities and ensure early reporting.
5. CEREBROSPINAL MENINGITIS (CSM)
5.7. In the reporting week 36, nine suspected Cerebrospinal Meningitis (CSM) cases were reported from seven LGAs (six States) compared with 26 suspected cases with one Laboratory-confirmed case from five LGAs (four States) at the same period in 2016.
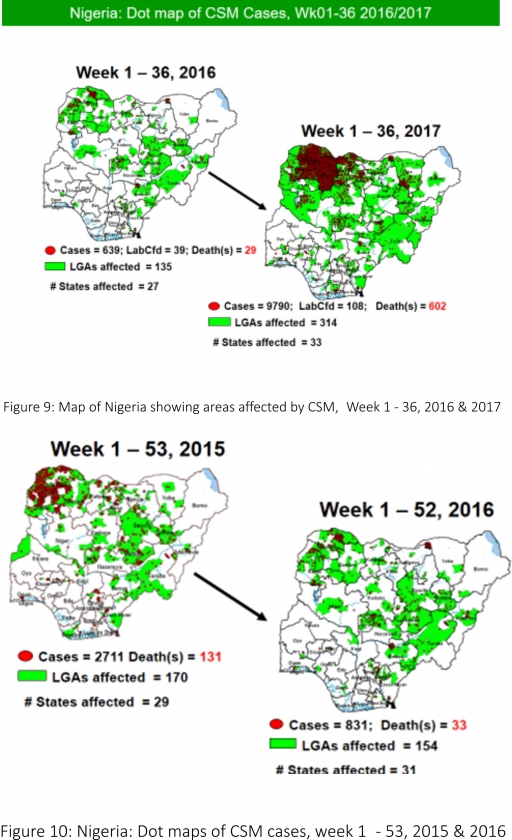
5.8. Between weeks 1 and 36 (2017), 9790 suspected CSM cases with 108 laboratory confirmed cases and 602 deaths (CFR, 6.15%) were recorded from 314 LGAs (33 States) compared with 639 suspected cases and 29 deaths (CFR, 4.54%) from 135 LGAs (27 States) during the same period in 2016 (Figure 9).
5.9. Between weeks 1 and 52, 2016, 831 suspected CSM cases with 43 laboratory confirmed cases and 33 deaths (CFR, 3.97%) were recorded from 154 LGAs (30 States and FCT) compared with 2,711 suspected cases and 131 deaths (CFR, 4.83%) from 170 LGAs (28 States and FCT) during the same period in 2015 (Figure 10)
5.10. Timeliness/completeness of CSM case-reporting from States to the National Level (2017 versus 2016): on average, 81.5% of the 26 endemic States sent CSM reports in a timely manner while 98.3% were complete in week 1 – 36, 2017 as against 85.3% timeliness and 98.5% completeness recorded within the same period in 2016
5.11. CSM preparedness checklist sent to 36 States and FCT ahead of 2017 meningitis season
5.12. Confirmed cases treated at identified treatment centres in affected States (Zamfara, Sokoto, Katsina, Kebbi, Niger, Kano, Yobe and Jigawa) and necessary supportive management also instituted
5.13. CSM National Emergency Operations Centre constituted at the Nigeria Centre for Disease Control
5.14. Onsite support provided to Zamfara, Sokoto, Katsina, Kebbi, Kano, Yobe and Niger States by NCDC and partners
5.15. Off-site support provided to other States
5.16. Intensive Surveillance in high-risk States and NCDC in communication with States reporting suspected cases.
5.17. Reactive vaccination completed in Zamfara, Sokoto and Yobe States
5.18. Medical teams were trained and deployed to support case management in Sokoto and the Zamfara States completed (from Friday 5th - 26th May 2017).
5.19. Deployed mobile testing laboratory to Zamfara State to aid diagnosis
5.20. A Team was deployed by NCDC/WHO to support surveillance activities, laboratory data harmonization and monitoring of the implementation plan in Yobe state
5.21. Evaluation of the CSM outbreak response in Zamfara and Sokoto States is ongoing by NCDC and WHO
5.22. National CSM After-Action Review meeting conducted in Sokoto State from the 24th – 25th of July 2017.
5.23. NCDC attended the 14th Annual Meeting on Surveillance, Preparedness and Response to Meningitis Outbreaks in Africa, and 4th Annual MenAfriNet Partners’meeting held in Ouagadougou, Burkina Faso in preparation of 2017/2018 meningitis season from 12th to 15th September 2017.
5.24. Ongoing finalization of the National CSM Guidelines
6. GUINEA WORM DISEASE
6.7. In the reporting week, no rumour report of Guinea Worm disease was received from any State.
6.8. Nigeria has celebrated eight consecutive years of zero reporting of Guinea worm disease in the country. The Country has been officially certified free of Dracunculiasis transmission by the International Commission for the Certification of Dracunculiasis Eradication (ICCDE).
(For further information, contact Nigeria Guinea Worm Eradication Program / Neglected Tropical Diseases Division, Public Health Department/Federal Ministry of Health)

7. Update on national Influenza sentinel surveillance, Nigeria week 1 - 36, 2017
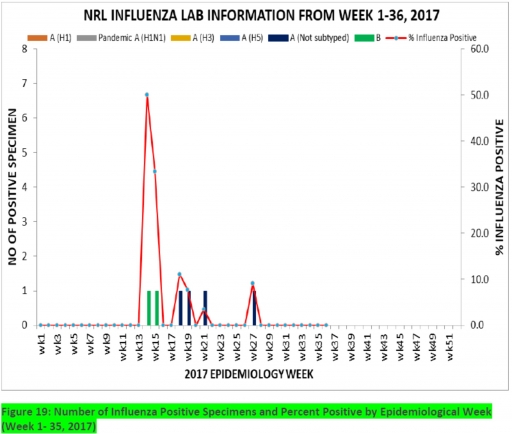
7.1. From week 1-36, a total of 103 cases were reported, of which 95 were Influenza-like-illness (ILI), 8 Severe Acute Respiratory Infection (SARI).
7.2 A total of 103 samples were received and all were processed. Of the processed samples, 95(92.2%) were ILI cases, 8(7.8%) were Severe Acute Respiratory Infection (SARI).
7.3 Of the 95 processed ILI samples, 4(4.2%) were positive for Influenza A; 2(2.1%) positive for Influenza B and 89(93.7%) were negative. Of the 8 processed SARI samples, (12.5%) was positive for Influenza A while 0(0.0%) was positive for Influenza B. 7(87.5%) were all negative. (not clear)
7.4. 6 (6.9%) of the processed 95 samples were positive for Influenza, with 4(66.7%) of these positive for Influenza A and 2(33.3%) positive for Influenza B. The subtypes A seasonal H3, 2009A/H1N1 and A/not subtyped account for (0.0%), 0(0.0%) and 2(50.0%) of the total influenza A positive samples respectively.
7.5. The percentage influenza positive was highest (50.0%) in week 14
7.6. In the reporting week36, no samples were left unprocessed
FOR MORE INFORMATION CONTACT
Surveillance Unit:
Nigeria Centre for Disease Control
801 Ebitu Ukiwe Street, Jabi, Abuja, Nigeria.
[email protected]
www.ncdc.gov.ng/reports
0800-970000-10










 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 