In Epi-week 38, active case search for suspected cases of yellow fever continued in Ifelodun Local Government Area of Kwara State and parts of Kogi State. Laboratory samples have been collected for testing and confirmation.
This week’s editorial is a second part in the control of yellow fever outbreak series and the focus is on case management of the disease.
There are several approaches to managing cases of Yellow fever, which is usually dependent on the phase of the illness. The severity of Yellow fever infection varies, as there are different phases of the disease. These phases and diagnostic criteria are described in the table below.
Infective o Experienced in about 10% of infected persons.
o Characterized by non-specific symptoms such as fever, muscle pain with prominent back pain, headache, loss of appetite, nausea and/or vomiting
o Infected persons recover from symptoms.
o Characterized by high-grade fever, jaundice (yellowing of the eyes and skin), dark urine and abdominal pain with vomiting.
There is no specific treatment for Yellow fever. Management of cases is usually symptomatic with supportive management provided. This includes:
1. Hospitalization of patients for supportive care and close observation. Severely ill patients may be treated in an intensive care setting, if available
4. Use of Pain relievers and medications to reduce fever and relieve aching symptoms. Certain medications should be avoided such as aspirin and non-steroidal anti-inflammatory drugs e.g. Ibuprofen, naproxen as they may increase the risk of bleeding
7. In severe case of bleeding, transfusion with fresh frozen plasma or use of heparin is indicated.
It is important to also protect the infected patient from further exposure to mosquitoes. This can be done by always staying indoors and/or under a mosquito net, for up to 5 days after the onset of fever. This will make them unavailable to uninfected mosquitoes thereby stopping the transmission cycle and further reduce risk of transmission to persons around them.
Outcome following a yellow fever infection may vary from mild to fatal. Majority of infected persons (about 80%) will be asymptomatic or have mild disease with complete recovery. The most important and critical step towards preventing an infection is vaccination, which is available from 9 months of age at health facilities.
The Nigeria Centre for Disease Control (NCDC) enjoins all states to be more vigilant and enhance their surveillance systems to be able to identify cases of yellow fever. States are also encouraged to sensitize and train healthcare workers on management procedures of yellow fever cases. Early reporting of suspected cases of Yellow fever to the next level is very important.
In the reporting week ending on the 24th of September, 2017:
o There were 224 new cases of Acute Flaccid Paralysis (AFP) reported. None was confirmed as Polio. The last reported case of Polio in Nigeria was in August 2016. Active case search for AFP is being intensified as Nigeria has assiduously reinvigorated its efforts at eradicating Polio.
o 107 suspected cases of Cholera were reported from six LGAs (five States; Bauchi – 4, Bayelsa – 8, Borno – 69, Kaduna – 8 and Kano – 18). No was laboratory confirmed and 2 deaths were recorded.
o 15 suspected cases of Lassa fever were reported from three LGAs in three States (Gombe – 2, Kaduna – 1 and Kwara – 12). No was laboratory confirmed and no death was recorded.
o There were seven suspected cases of Cerebrospinal Meningitis (CSM) reported from seven LGAs in six States (Benue – 1, Borno – 1, Ebonyi – 1, Enugu – 1, Gombe – 1 and Katsina - 2). Of these, none was laboratory confirmed and no death was recorded. Ongoing surveillance for CSM has been intensified in all the 26 States in the Nigeria meningitis belt.
o There were 265 suspected cases of Measles reported from 26 States. None was laboratory confirmed and no death was recorded.
In the reporting week, all States sent in their report. This is a remarkable improvement! Timeliness of reporting remains 84% in both previous and current weeks (Week 37 and 38) while completeness remains at 100%. It is very important for all States to ensure timely and complete reporting at all times, especially during an outbreak.
1. Lassa fever
Please note that the data reflects the routine reports i.e. all suspected cases including the laboratory positive and negative cases
1.1. 15 suspected cases of Lassa fever were reported from three LGAs (three States; Gombe – 2, Kaduna – 1 and Kwara – 12) in week 38, 2017 compared with three suspected cases reported from three LGAs (two States) at the same period in 2016.
1.2. Laboratory results of the 15 suspected cases were three negative for Lassa fever and other VHFs (Gombe – 2 and Kaduna - 1) while that of Kwara State (12) is still pending.
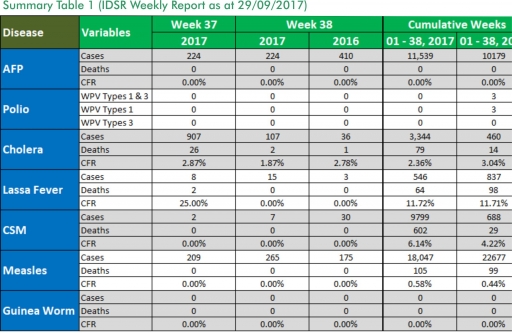
1.3. Between weeks 1 and 38 (2017), 546 suspected Lassa fever cases with 117 laboratory confirmed cases and 64 deaths (CFR, 11.72%) from 80 LGAs (26 States) were reported compared with 837 suspected cases with 83 laboratory confirmed cases and 98 deaths (CFR, 11.71%) from 135 LGAs (28 States) during the same period in 2016 (Figure 1).
1.4. Between weeks 1 and 52 2016, 921 suspected Lassa fever cases with 109 laboratory confirmed cases and 119 deaths (CFR, 12.92%) from 144 LGAs (28 States and FCT) were reported compared with 430 suspected cases with 25 laboratory confirmed cases and 40 deaths (CFR, 9.30%) from 37 LGAs (14 States and FCT) during the same period in 2015 (Figure 2).
1.5. Investigation and active case search ongoing in affected States with coordination of response activities by the NCDC with support from partners.
1.5.1. National Lassa Fever Working Group meeting and weekly National Surveillance and Outbreak Response meeting on-going at NCDC to keep abreast of the current Lassa fever situation in the country.
1.5.2. Response materials for VHFs provided to support States
1.5.3. New VHF guidelines have been developed by the NCDC (National Viral Haemorrhagic Fevers Preparedness guidelines, Infection Prevention and Control of VHF and Standard Operating Procedures for Lassa fever management) and are available on the NCDC website.
1.5.4. VHF case-based forms completed by affected States are being entered into the new VHF management system. This system allows for the creation of a VHF database for the country.
1.5.5. Confirmed cases are being treated at identified treatment/isolation centres across the States with Ribavirin and necessary supportive management also instituted
1.5.6. Onsite support was earlier provided to Ogun, Nasarawa, Taraba, Ondo and Borno States by the NCDC and partners
1.5.7. Offsite support provided by NCDC/partners in all affected States
1.5.8. States are enjoined to intensify surveillance and promote Infection, Prevention and Control (IPC) measures in health facilities.
2. MEASLES
2.1. In the reporting week, 265 suspected cases of Measles were reported from 26 States compared with 175 suspected cases reported from 24 States during the same period in 2016.
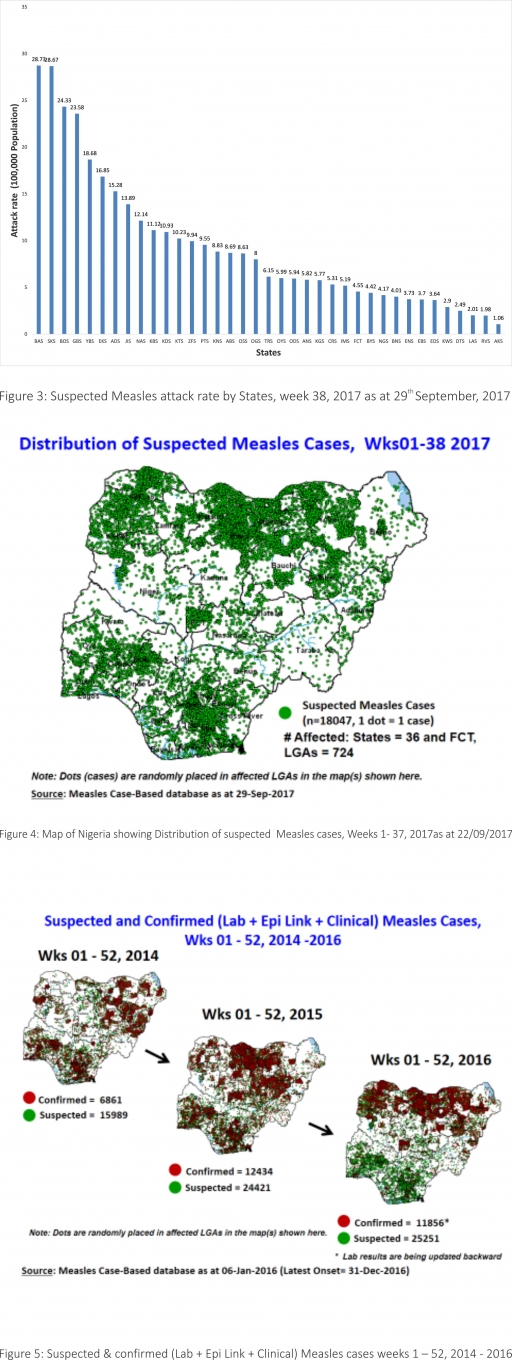
2.2. So far, 18,047 suspected Measles cases with 108 laboratory confirmed cases and 105 deaths (CFR, 0. 59%) have been reported in 2017 from 36 States and FCT (Figure 4) compared with 22,677 suspected cases and 99 deaths (CFR, 0.44%) from 36 States and FCT during the same period in 2016.
2.3. In 2016 (week 1 -52), 25,251 suspected Measles cases with 102 deaths (CFR, 0.40%) were reported from 36 States and FCT compared with 24,421 suspected cases with 127 deaths (CFR, 0.52%) during the same period in 2015 (Figure 5)
2.4. Response measures include immunisation for all vaccine-preventable diseases in some selected/affected wards/LGAs during SIAs, as well as case management.
2.5. Scheduled Measles campaigns in the North East were conducted from 12th – 17th January 2017 in Adamawa, Borno and Yobe States (Phase I) and Phase II from 21st – 25th January 2017 in Borno State and 4th – 8th February 2017 in Yobe State
2.6. Measles Surveillance Evaluation and Establishment of the burden of Congenital Rubella Syndrome (CRS) in 12 selected States in the six geopolitical zones from the 17th -21st July 2017 conducted
2.6.1 Debrief meeting to review results and next steps from Measles evaluation conducted, held on the 15th of September 2017
2.7. Harmonisation of measles surveillance data with laboratory-confirmed cases
3. POLIOMYELITIS
3.1. As at September 17th, 2017, no new case of WPV was recorded
3.2. Three new cVDPV2, environmental derived and Polio compatible cases identified
3.2.1. In the reporting week, 224 cases of AFP were reported from 174 LGAs in 28 States and FCT
3.2.2. AFP Surveillance has been enhanced and outbreak response is on-going in Borno and other high-risk States
3.2.3. The 1st round of SIPDs in 2017 was conducted from 28th – 31st January 2017 in the 18 high-risk States. This was carried out using mOPV2 (2nd mOPV2 OBR). The schedule for other SIAs is as described in Table 2
3.2.4. The 2nd and 3rd round of SIPDs completed (25th-28th February and 8th – 11th July 2017) in 14 & 18 high-risk States using bOPV respectively.
3.2.5. The 1st and 2nd rounds of NIPDs completed (from 25th – 28th March 2017 and 22nd – 25th April 2017) nationwide respectively.
3.2.6. Between weeks 1 and 52 in 2016, four WPVs were isolated from Borno State compared to no WPV isolated during the same period in 2015.
3.3. No circulating Vaccine Derived Polio Virus type 2 (cVDPV2) was isolated in week 1 - 52, in both 2016 and 2015.
3.4. Between weeks 1 and 52, 2016 two (2) cVDPV2 were isolated in two LGAs (two States) while one (1) cVDPV2 was isolated from Kwali, FCT during the same period in 2015.
3.5. Six confirmed WPVs were isolated in 2014.
3.6. The SIAs were strengthened with the following events:
3.6.1. Immunisation for all vaccine-preventable diseases in some selected wards/LGAs.
3.6.2. Use of health camp facilities.
3.6.3. Field supportive supervision and monitoring.
3.6.4. Improved Enhanced Independent Monitoring (EIM) and Lots Quality Assessments (LQAs) in all Polio high-risk States.
3.6.5. High level of accountability framework
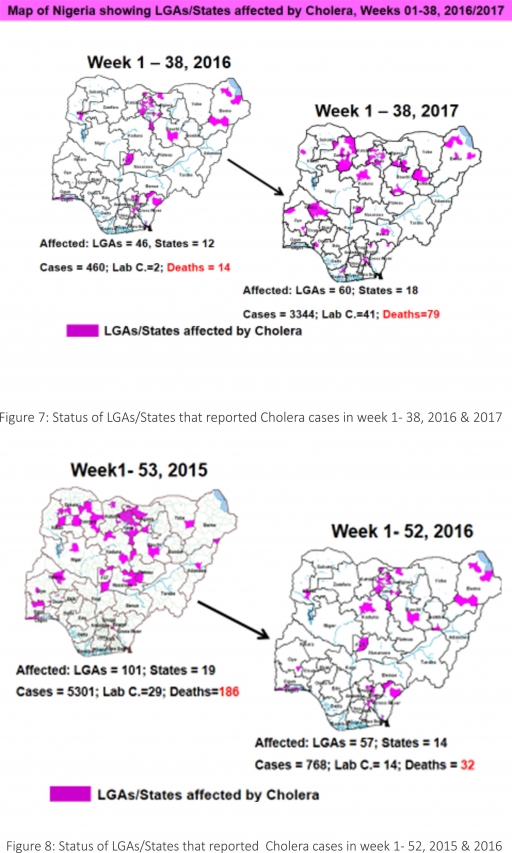
4. CHOLERA
4.1. 107 suspected cases of Cholera and 2 deaths (CFR, 2.87%) were reported from six LGAs (five States; Bauchi – 4, Bayelsa – 8, Borno – 69, Kaduna - 8 and Kano – 18) in week 38 compared with 35 suspected cases and one Laboratory-confirmed case reported from 13 LGAs in three States during the same period in 2016.
4.2. Between weeks 1 and 38 (2017), 3344 suspected Cholera cases with 41 laboratory confirmed and 79 deaths (CFR, 2.38%) from 60 LGAs (18 States) were reported compared with 460 suspected cases and 14 deaths (CFR, 3.04%) from 46 LGAs (12 States) during the same period in 2016 (Figure 7).
4.3. Between weeks 1 and 52 (2016), 768 suspected Cholera cases with 14 laboratory confirmed cases and 32 deaths (CFR, 4.17%) from 57 LGAs (14 States) were reported compared with 5,301 cases with 29 laboratory confirmed cases and 186 deaths (CFR, 3.51%) from 101 LGAs (18 States and FCT) during the same period in 2015 (Figure 8).
4.4. Cholera preparedness workshop held from 31st May – 1st June 2017 in Abuja to
develop Cholera preparedness plan as the season set in.
4.5. NCDC/partners provided onsite support in Kwara, Zamfara and Kebbi States.
4.6 NCDC/partners are providing onsite support in Borno State.
4.7. Preparedness and Response to Acute Watery Diarrhoea/ Cholera Guidelines have been finalised http://ncdc.gov.ng/themes/common/docs/protocols/45_1507196550.pdf
4.8. RDT procured by NCDC and WHO currently being prepositioned in affected States
4.9. States are enjoined to intensify surveillance, implement WASH activities and ensure early reporting.
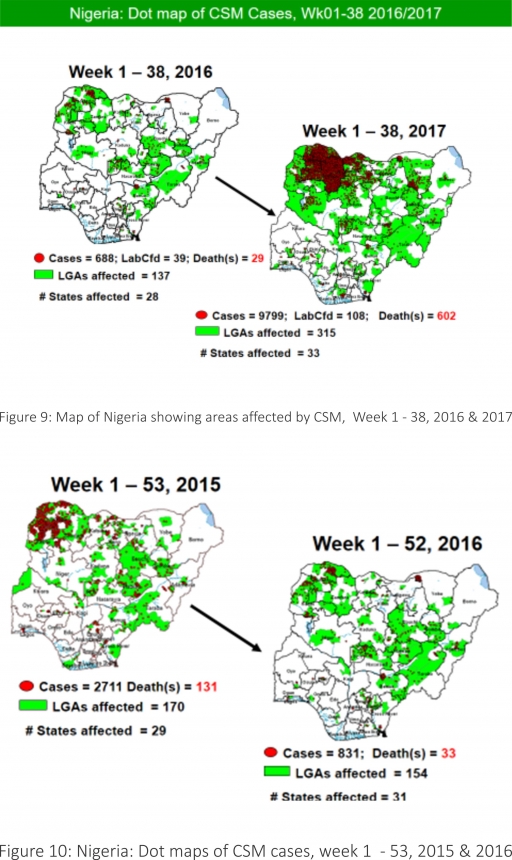
5. CEREBROSPINAL MENINGITIS (CSM)
5.7. In the reporting week 38, seven suspected Cerebrospinal Meningitis (CSM) cases were reported from seven LGAs (six States) compared with 30 suspected cases from seven LGAs (six States) at the same period in 2016.
5.8. Between weeks 1 and 38 (2017), 9799 suspected CSM cases with 108 laboratory confirmed cases and 602 deaths (CFR, 6.15%) were recorded from 315 LGAs (33 States) compared with 688 suspected cases and 29 deaths (CFR, 4.22%) from 137 LGAs (28 States) during the same period in 2016 (Figure 9).
5.9. Between weeks 1 and 52, 2016, 831 suspected CSM cases with 43 laboratory confirmed cases and 33 deaths (CFR, 3.97%) were recorded from 154 LGAs (30 States and FCT) compared with 2,711 suspected cases and 131 deaths (CFR, 4.83%) from 170 LGAs (28 States and FCT) during the same period in 2015 (Figure 10)
5.10. Timeliness/completeness of CSM case-reporting from States to the National Level (2017 versus 2016): on average, 81.9% of the 26 endemic States sent CSM reports in a timely manner while 99.1% were complete in week 1 – 38, 2017 as against 85.8% timeliness and 99.9% completeness recorded within the same period in 2016
5.11. CSM preparedness checklist sent to 36 States and FCT ahead of 2017 meningitis season
5.12. Confirmed cases treated at identified treatment centres in affected States (Zamfara, Sokoto, Katsina, Kebbi, Niger, Kano, Yobe and Jigawa) and necessary supportive management also instituted
5.13. CSM National Emergency Operations Centre constituted at the Nigeria Centre for Disease Control
5.14. Onsite support provided to Zamfara, Sokoto, Katsina, Kebbi, Kano, Yobe and Niger States by NCDC and partners
5.15. Off-site support provided to other States
5.16. Intensive Surveillance in high-risk States and NCDC in communication with States reporting suspected cases.
5.17. Reactive vaccination completed in Zamfara, Sokoto and Yobe States
5.18. Medical teams were trained and deployed to support case management in Sokoto and Zamfara States completed (from Friday 5th - 26th May 2017).
5.19. Deployed mobile testing laboratory to Zamfara State to aid diagnosis
5.20. A Team was deployed by NCDC/WHO to support surveillance activities, laboratory data harmonization and monitoring of the implementation plan in Yobe state
5.21. NCDC attended the 14th Annual Meeting on Surveillance, Preparedness and Response to Meningitis Outbreaks in Africa, and 4th Annual MenAfriNet Partners’ meeting held in Ouagadougou, Burkina Faso in preparation of 2017/2018 meningitis season from 12th to 15th September 2017.
5.22. Ongoing finalisation of the National CSM Guidelines
6. GUINEA WORM DISEASE
6.7. In the reporting week, no rumour report of Guinea Worm disease was received from any State.
6.8. Nigeria has celebrated eight consecutive years of zero reporting of Guinea worm disease in the country. The Country has been officially certified free of Dracunculiasis transmission by the International Commission for the Certification of Dracunculiasis Eradication (ICCDE).
(For further information, contact Nigeria Guinea Worm Eradication Program / Neglected Tropical Diseases Division, Public Health Department/Federal Ministry of Health)

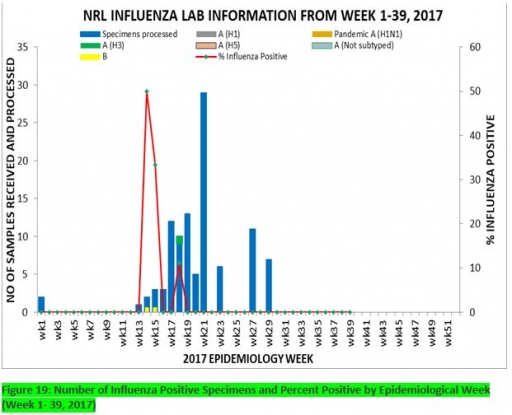
7. Update on national Influenza sentinel surveillance, Nigeria week 1 - 39, 2017
7.1. From week 1-39, a total of 103 suspected cases were reported, of which 95 were Influenza-like-illness (ILI), 8 Severe Acute Respiratory Infection (SARI).
7.2 A total of 103 samples were received and all were processed. Of the processed samples, 95(92.2%) were ILI cases, 8(7.8%) were Severe Acute Respiratory Infection (SARI).
7.4. Of the 95 processed ILI samples, 1(1.05%) was positive for Influenza A; 2(2.1%) positive for Influenza B and 92(98.95%) were negative. Of the 8 processed SARI samples, none was positive for Influenza A and Influenza B.
7.5. AIn the reporting week 39, no samples were left unprocessed










 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 