Editoral
Nigeria Conducts Mid-term Joint External Evaluation of International Health Regulations Capacities
Posted: 26-11-2019 03:14:02 PM
In 2017, Nigeria voluntarily joined the list of countries to conduct the Joint External Evaluation (JEE) of International Health Regulations (IHR, 2005) capacities. To address the critical gaps identified following Nigeria’s JEE, the Nigeria Centre for Disease Control (NCDC) as the IHR National Focal Point together with various Ministries, Departments and Agencies (MDAs), supported by partners, developed a National Action Plan for Health Security (NAPHS). The NAPHS is a five-year multi-sectoral and overarching strategic plan intended to provide clear roadmap for implementation of activities to address the JEE gaps.
Two years after the first JEE, relevant MDAs and partners whose functions are related to national health security identified the need to review progress made in the 19 technical areas as well as areas for prioritisation to meet the 2022 target set during the first JEE. To achieve this, a mid-term JEE was conducted from 18th -22nd November, 2019 using the World Health Organization (WHO) approved JEE 2.0 tool.
The process included an initial internal assessment by relevant MDAs and an external validation by a mission Team which included representatives from partner organisations. The major outputs from the meeting were:
1. Review of activities in the 19 technical areas since the first JEE and progress made
2. Prioritised activities matched with resource needs for implementation
3. Improved collaboration and coordination among MDAs with functions related to national health security
The Honourable Minister of Health, Dr. Osagie Ehanire, received the results of the JEE from the mission Team. He highlighted the commitment and support from His Excellency, President Muhammadu Buhari for to strengthen national health security. While congratulating Nigeria on the successful review, the Officer in Charge of WHO Nigeria, Dr. Clement Peter-Lasuba, attributed significant progress recorded to strong partnerships and increasing multi-sectoral approach.
In concluding, the Director General of NCDC, Dr. Chikwe Ihekweazu, emphasised the need for improved collaboration among the various MDAs involved in national health security.
Summary of Incidents
Notes
1. Information for this disease was retrieved from the Technical Working Group and Situation Reports
2. Case Fatality Rate (CFR) for this disease is reported for confirmed cases only
3. Information for this disease was retrieved from IDSR 002 data
4. CFR for this disease is reported for total cases i.e. suspected + confirmed
5. Information for sentinel influenza was retrieved from the laboratory
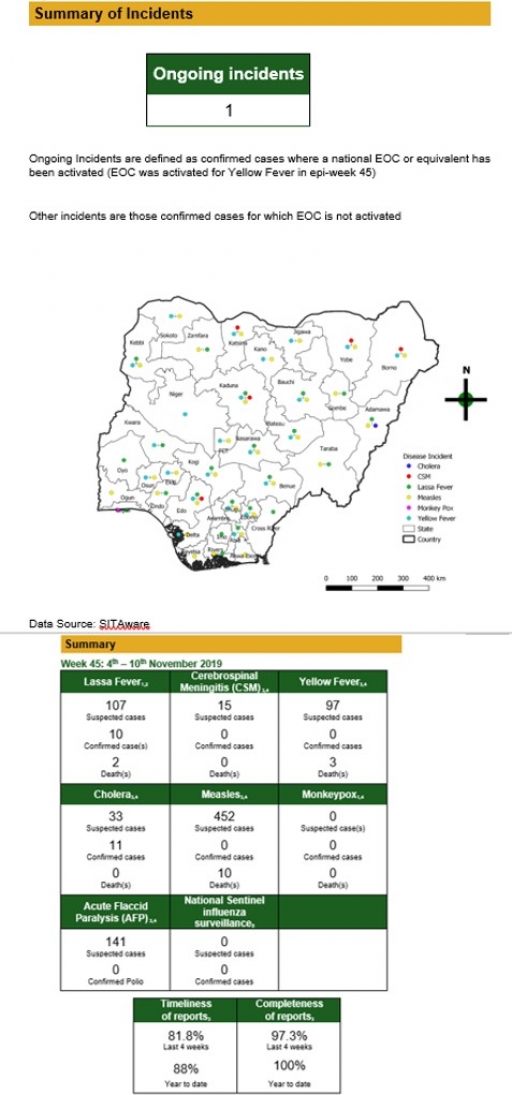
Lassa Fever
Key points
• There were 107 suspected cases of Lassa Fever (LF) reported from 17 LGAs in 13 states (Edo – 53, Ondo – 23, Ebonyi – 6, Bauchi – 12, Nasarawa – 1, Plateau – 4, Taraba – 1, Gombe – 1, Benue – 1, Kogi – 1, Ogun – 1, Lagos - 1 & Abia – 2). There were ten confirmed cases and two deaths were recorded (Edo and Abia states)
Actions
To date:
• The national Lassa Fever multi-sectoral Technical Working Group (TWG) continues to coordinate response activities and support States
• Lassa Fever National Environmental Response was conducted in Edo and Ondo states: Community Rodent Control, environmental sanitation and personal hygiene promotion. This was coordinated by Federal Ministry of Environment (FMEnv) in collaboration with NCDC and supported by WHO
Planned:
• Finalise advocacy packages for national and subnational policy/decision makers ahead of ‘Lassa fever’ season
• Finalise the Lassa fever five-year strategic plan
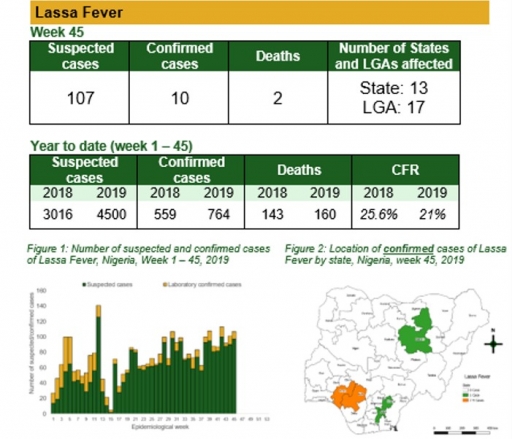
Cerebrospinal Meningitis (CSM)
Key points
There were 15 suspected cases of Cerebrospinal Meningitis (CSM) reported from nine LGAs in three states (Borno – 1, Katsina – 10 & Yobe – 4). None was laboratory confirmed and no death was recorded
Actions
To date:
• The national CSM Technical Working Group (TWG) meets weekly to review reports from states and plan appropriately
Planned:
• Continue harmonisation of the national line list and SORMAS data
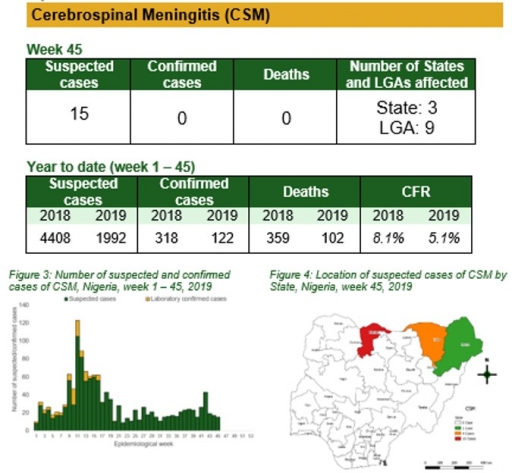
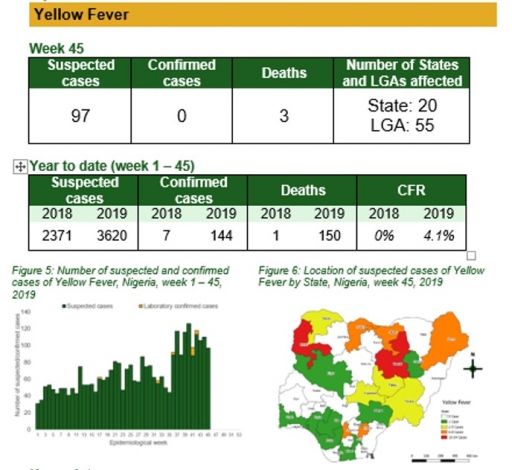
Yellow Fever
Key points
• There were 97 suspected cases of Yellow Fever (YF) reported from 55 LGAs in 20 states. None was laboratory confirmed and three deaths were recorded
• Follow up with the new states with confirmed cases
Actions
To date:
• The national YF Technical Working Group to continues to coordinate response activities
Planned:
• Follow up with NPHCDA on the pre-implementation plans for YF preventive/reactive mass vaccination campaigns in the implementing LGAs/states
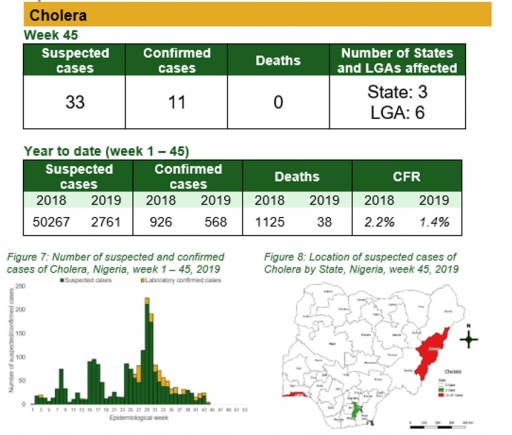
Cholera
Key points
• There were 33 suspected cases of cholera reported from three LGAs in six states. There were 11 laboratory confirmed cases and no death was recorded
Actions
To date
• The national cholera multi-sectoral Technical Working Group (TWG) is monitoring all states and supporting already affected states
• Communication team working with relevant TWGs to develop flood advisories
Planned:
• Follow up with states with active outbreaks and monitor non-reporting states
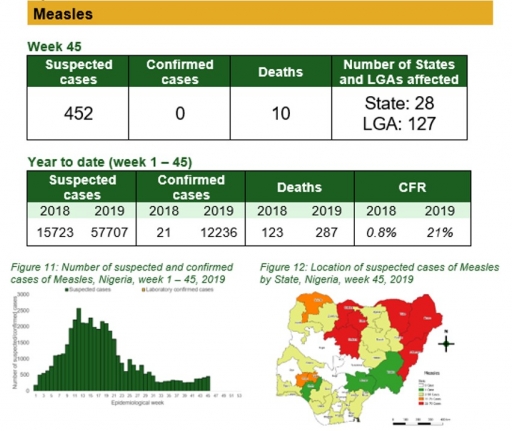
Measles
Key points
• There were 452 suspected cases of measles reported from 127 LGAs in 28 states. None was laboratory confirmed and ten deaths was recorded
Actions
To date
• The national measles Technical Working Group (TWG) is closely monitoring surveillance data and response activities across the country
Planned:
• Continue review of measles surveillance data across the country
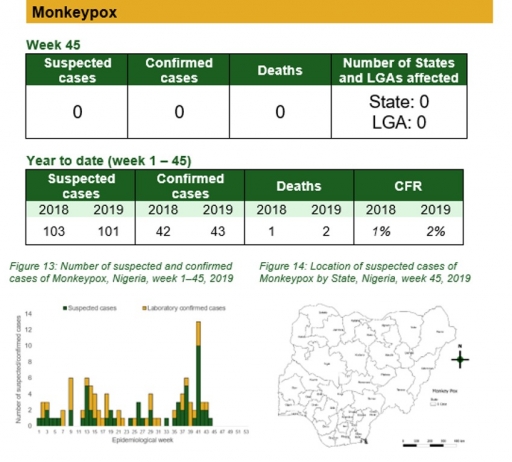
Monkeypox
Key points
• There were no suspected cases of monkeypox reported this week.
Actions
• The national Monkeypox Technical Working Group (TWG) is monitoring activities in all states
Planned
• Regional monkeypox surveillance training in South East, South West and North Central in November 2019
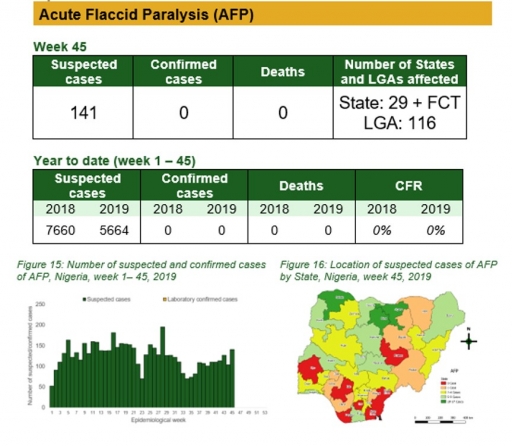
Acute Flaccid Paralysis (AFP)
Key points
• There were 141 suspected cases of AFP reported from 116 LGAs in 29 states and FCT. None was laboratory confirmed and no death was recorded
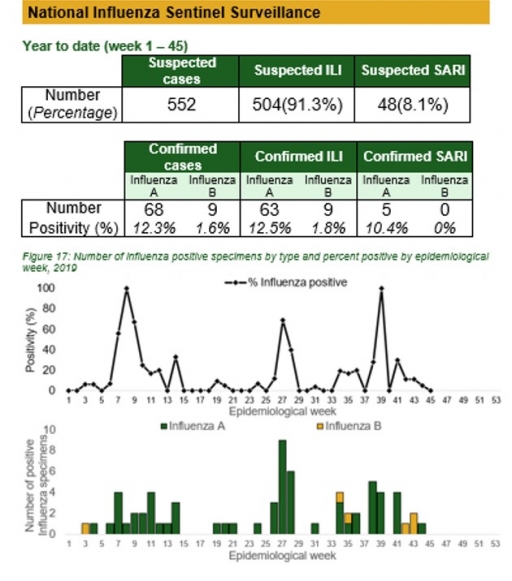
National Influenza Sentinel Surveillance
Key points
There were 78 processed samples positive for influenza, with 68 for influenza A, 9 for influenza B and 1 for influenza A & B
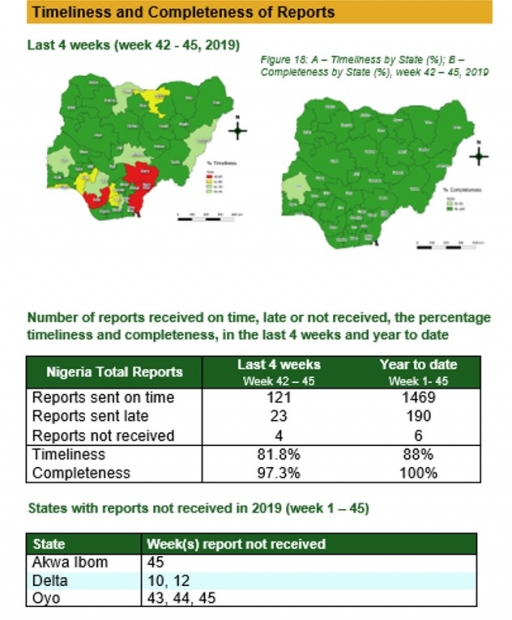
Timeliness and Completeness of Reports
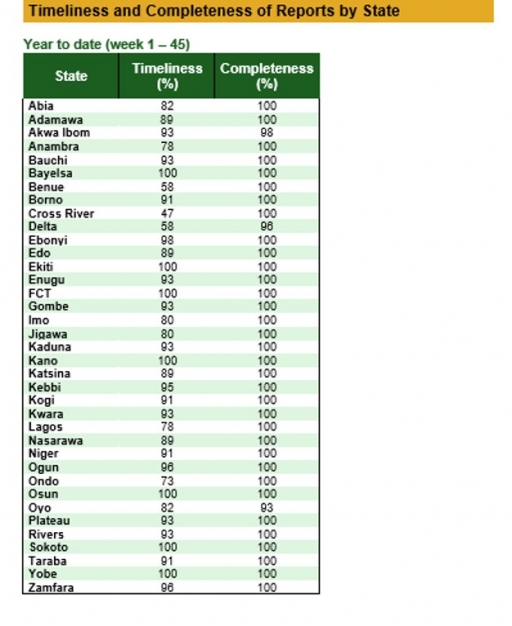
Timeliness and Completeness of Reports by State













 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 