As the dry season begins, we are strengthening preparedness for disease outbreaks especially for diseases with more cases recorded during this time of the year. With a focus on Cerebrospinal Meningitis last week, we discuss outbreak preparedness activities specific for Lassa fever and what is expected at each level of Government, in this week’s report. These are discussed under six pillars: Surveillance, Case management, Laboratory, Logistics, Risk Communication/Social Mobilisation and Coordination
• Training of community informants on case detection for Lassa fever using the standard case definition to identify alert and suspected cases
• Training and mentoring for health facility surveillance focal persons on surveillance and reporting
• Support training and mentoring for health facility surveillance focal persons on surveillance and reporting
• Support states in surveillance activities through the use of the event-based surveillance system.
• Ensuring holding areas are earmarked in health facilities.
• Advisories to be shared to treatment centres and health facilities on imminent Lassa fever season.
• Distribution of National guidelines on Lassa fever case management and IPC guidelines to health facilities
• Commence purchase and distribution of Ribavirin, Personal Protective Equipment(PPE) and other consumables for Lassa fever management using previous state-specific epidemiological data
• Notification to state Emergency Preparedness response team on imminent Lasa fever outbreak
• Stockpiling of PPEs, ribavirin and other commodities as supplementary stock for States
• Refresher training and mentoring sessions for healthcare workers on sample collection for Lassa fever
• LGA DSNO to ensure sample collection kits are available and within validity period
• Ensure all materials needed for sample collection are available and within validity period
• Commence purchase of small quantities of triple packaging kits in readiness for an outbreak, especially for Lassa fever prone states
• Commence negotiations with courier delivery services for sample transportation
• Ensure resources required for case investigation, monitoring, sample transportation etc are available
• Commence purchase of small quantities of ribavirin, PPEs and other consumables, using previous epidemiological data
• Commence purchase of small quantities of ribavirin, PPEs and other consumables, as supplementary stock
• Commence production of IEC materials (preferably in local languages) for distribution to the LGAs and communities
• Commence production and broadcast of TV and radio jingles on Lassa fever. Information shared to highlight the signs/symptoms and prevention measures to be taken. Contact details of relevant offices for notification to be shared with the general public
• Carry out sensitization campaigns through TV and radio interviews as well as use on state social media platforms.
• Identify any public health activity which can be leveraged on for increased coverage of campaign reach for Lassa fever
• Support sensitization activities with messages shared via mass, print and social media
• Advocacy to relevant stakeholders to garner support and commitment to Lassa fever outbreak
• National Lassa fever working group to commence coordination of preparedness activities
The Nigeria Centre for Disease Control (NCDC) is deploying three teams to visit three high priority state to assess the level of state preparedness for the imminent Lassa fever season and provide support as required. This is in addition to other critical preparedness activities taking place. States are encouraged to commence preparedness activities to ensure improved health outcomes during the outbreak season.
In the reporting week ending on the 19th of November, 2017:
o There were 199 new cases of Acute Flaccid Paralysis (AFP) reported. None was confirmed as Polio. The last reported case of Polio in Nigeria was in August 2016. Active case search for AFP is being intensified as Nigeria has reinvigorated its efforts at eradicating Polio.
o Ten suspected cases of Cholera were reported from three LGAs in three States (Bauchi – 1, Borno – 1 and Kaduna – 8). None was laboratory confirmed and no death was recorded.
o 24 suspected cases of Lassa fever were reported from eight LGAs in (five States: Bauchi – 2, Edo – 18, Kaduna – 1, Oyo -1 & Plateau - 2). Three were laboratory confirmed and no death was recorded.
o There were 22 suspected cases of Cerebrospinal Meningitis (CSM) reported from 11 LGAs in eight States (Anambra – 1, Cross River – 6, Kaduna – 1, Katsina -2, Lagos – 1, Ondo – 1, Oyo – 2 & Sokoto - 8). Of these, none was laboratory confirmed and no death was recorded. Ongoing surveillance for CSM has been intensified in all the 26 States in the Nigeria meningitis belt and to commence case-based surveillance from 4th December 2017.
o There were 265 suspected cases of Measles reported from 33 States. None was laboratory confirmed and one death was recorded.
In the reporting week, all States sent in their report. This is a remarkable improvement! Timeliness of reporting remains 85% in both previous and current weeks (Week 44 and 45) while completeness remains at 100%. It is very important for all States to ensure timely and complete reporting at all times, especially during an outbreak.
1. LASSA FEVER
Please note that the data reflects the routine reports i.e. all suspected cases including the laboratory positive and negative cases
1.1. 24 suspected cases of Lassa fever with three Laboratory confirmed were reported from eight LGAs (five States: Bauchi – 2, Edo – 18, Kaduna – 1, Oyo -1 & Plateau - 2) in week 46, 2017 compared with six suspected cases reported from four LGAs (three States) at the same period in 2016
1.2. Laboratory results of the 24 suspected cases; three positive for Lassa fever (Bauchi – 1, Kaduna – 1 & Plateau - 1) and 21 were negative for Lassa fever & other VHFs (Bauchi -1, Edo – 18, Oyo – 1 & Plateau – 1)
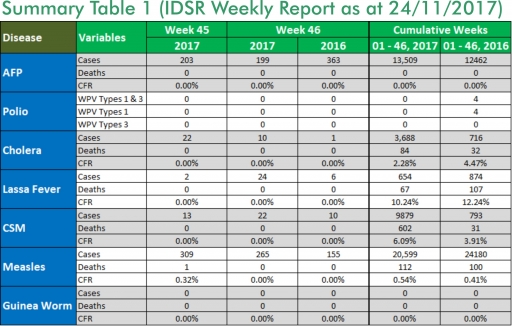
1.3. Between weeks 1 and 46 (2017), 654 suspected Lassa fever cases with 129 laboratory confirmed cases and 67 deaths (CFR, 10.24%) from 94 LGAs (27 States) were reported compared with 874 suspected cases with 89 laboratory confirmed cases and 107 deaths (CFR, 12.24%) from 141 LGAs (29 States) during the same period in 2016 (Figure 1)
1.4. Between weeks 1 and 52 2016, 921 suspected Lassa fever cases with 109 laboratory confirmed cases and 119 deaths (CFR, 12.92%) from 144 LGAs (28 States and FCT) were reported compared with 430 suspected cases with 25 laboratory confirmed cases and 40 deaths (CFR, 9.30%) from 37 LGAs (14 States and FCT) during the same period in 2015 (Figure 2)
1.5. Investigation and active case search ongoing in affected States with coordination of response activities by the NCDC with support from partners
1.5.1. National Lassa Fever Working Group meeting and weekly National Surveillance and Outbreak Response meeting on-going at NCDC to keep abreast of the current Lassa fever situation in the country
1.5.2. Response materials for VHFs provided to support States
1.5.3. New VHF guidelines have been developed by the NCDC (National Viral Haemorrhagic Fevers Preparedness guidelines, Infection Prevention and Control of VHF and Standard Operating Procedures for Lassa fever management) and are available on the NCDC website- http://ncdc.gov.ng/diseases/guidelines
1.5.4. VHF case-based forms completed by affected States are being entered into the new VHF management system. This system allows for the creation of a VHF database for the country. Data from the VHF database is currently being analysed to inform decision making in the coming year
1.5.5. Confirmed cases are being treated at identified treatment/isolation centres across the States with Ribavirin and necessary supportive management also instituted
1.5.6. Onsite support was earlier provided to Ogun, Nasarawa, Taraba, Ondo and Borno States by the NCDC and partners
1.5.7. Offsite support provided by NCDC/partners in all affected States
1.5.8. States are enjoined to intensify surveillance and promote Infection, Prevention and Control (IPC) measures in health facilities
1.5.9. Ongoing plans to support priority States in developing preparedness and response plans ahead of dry season
1.6.0 Plan to visit Bauchi, Ebonyi and Taraba States to assess their level of preparedness from 4th December 2017
2. MEASLES
2.1. In the reporting week, 265 suspected cases of Measles were reported from 33 States compared with 155 suspected cases reported from 27 States during the same period in 2016
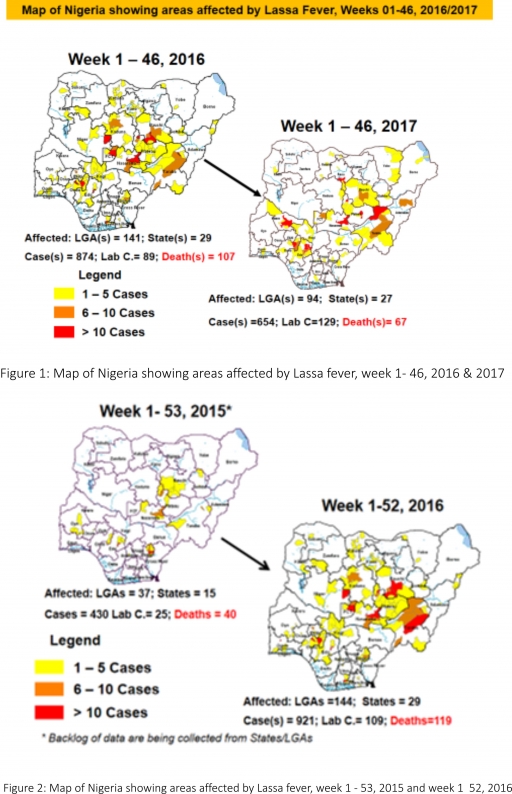
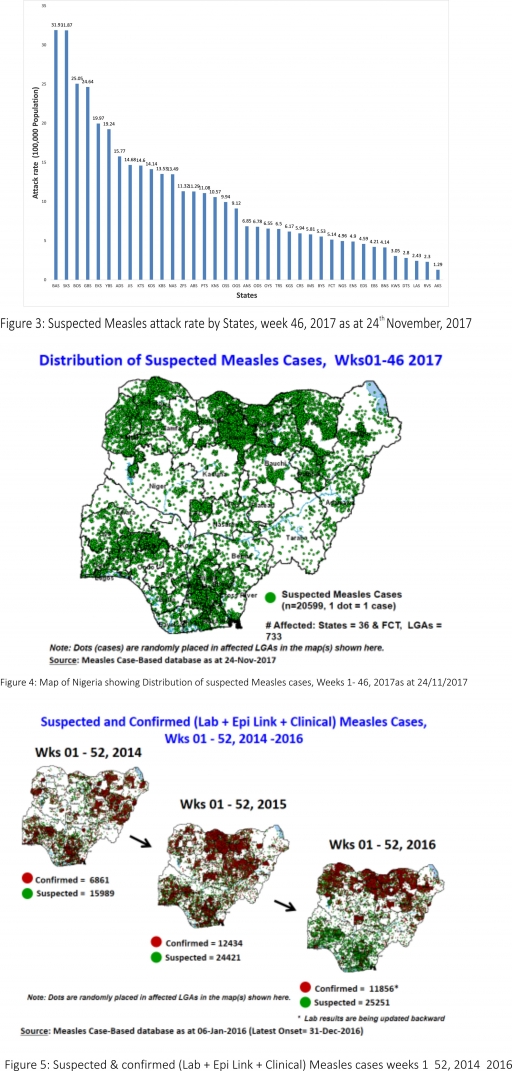
2.2. So far, 20,599 suspected Measles cases with 108 laboratory confirmed cases and 112 deaths (CFR, 0. 54%) have been reported in 2017 from 36 States and FCT (Figure 4) compared with 24,180 suspected cases and 100 deaths (CFR, 0.41%) from 36 States and FCT during the same period in 2016
2.3. In 2016 (week 1 -52), 25,251 suspected Measles cases with 102 deaths (CFR, 0.40%) were reported from 36 States and FCT compared with 24,421 suspected cases with 127 deaths (CFR, 0.52%) during the same period in 2015 (Figure 5)
2.4. Response measures include immunization for all vaccine-preventable diseases in some selected/affected wards/LGAs during SIAs, as well as case management
2.5. Scheduled Measles campaigns in the North East were conducted from 12th – 17th January 2017 in Adamawa, Borno and Yobe States (Phase I) and Phase II from 21st – 25th January 2017 in Borno State and 4th – 8th February 2017 in Yobe State
2.6. Measles Surveillance Evaluation and Establishment of the burden of Congenital Rubella Syndrome (CRS) in 12 selected States in the six geopolitical zones from the 17th -21st July 2017 conducted
2.7. Measles mass campaign conducted in seven North West States and ongoing in the North East States
3. POLIOMYELITIS
3.1. As at November 17th, 2017, no new case of WPV was recorded
3.2. Three new cVDPV2, environmental derived and Polio compatible cases identified
3.2.1. In the reporting week, 199 cases of AFP were reported from 168 LGAs in 36 States and FCT
3.2.2. AFP Surveillance has been enhanced and outbreak response is on-going in Borno and other high-risk States
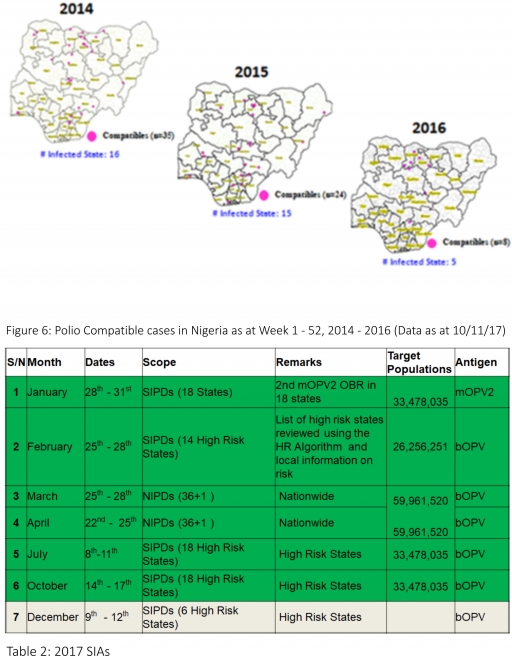
3.2.3. The 1st round of SIPDs in 2017 was conducted from 28th – 31st January 2017 in the 18 high-risk States. This was carried out using mOPV2 (2nd mOPV2 OBR). The schedule for other SIAs is as described in Table 2
3.2.4. The 2nd and 3rd round of SIPDs completed (25th-28th February and 8th – 11th July 2017) in 14 & 18 high-risk States using bOPV respectively.
3.2.5. The 1st and 2nd rounds of NIPDs completed (from 25th – 28th March 2017 and 22nd – 25th April 2017) nationwide respectively.
3.2.6. The 4th round of SIPDs completed from 14th- 17th October 2017 in 18 high-risk States using bOPV.
3.2.7. Between weeks 1 and 52 in 2016, four WPVs were isolated from Borno State compared to no WPV isolated during the same period in 2015.
3.3. No circulating Vaccine Derived Polio Virus type 2 (cVDPV2) was isolated in week 1 - 52, in both 2016 and 2015.
3.4. Between weeks 1 and 52, 2016 two (2) cVDPV2 were isolated in two LGAs (two States) while one (1) cVDPV2 was isolated from Kwali, FCT during the same period in 2015.
3.5. Six confirmed WPVs were isolated in 2014.
3.6. The SIAs were strengthened with the following events:
3.6.1. Immunisation for all vaccine-preventable diseases in some selected wards/LGAs.
3.6.2. Use of health camp facilities.
3.6.3. Field supportive supervision and monitoring.
3.6.4. Improved Enhanced Independent Monitoring (EIM) and Lots Quality Assessments (LQAs) in all Polio high-risk States.
3.6.5. High level of accountability framework
4. CHOLERA
4.1. Ten suspected cases of Cholera were reported from three LGAs (three States; Bauchi – 1, Borno – 1, & Kaduna -8) in week 46 compared with one suspected case reported from Gombe LGA (Gombe State) during the same period in 2016.
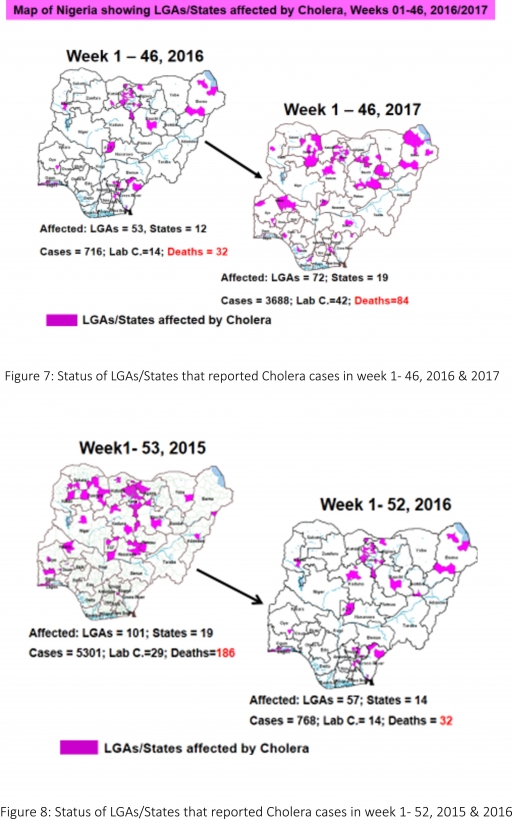
4.2. Between weeks 1 and 46 (2017), 3688 suspected Cholera cases with 42 laboratory confirmed and 84 deaths (CFR, 2.28%) from 72 LGAs (19 States) were reported compared with 716 suspected cases and 32 deaths (CFR, 4.47%) from 53 LGAs (12 States) during the same period in 2016 (Figure 7).
4.3. Between weeks 1 and 52 (2016), 768 suspected Cholera cases with 14 laboratory confirmed cases and 32 deaths (CFR, 4.17%) from 57 LGAs (14 States) were reported compared with 5,301 cases with 29 laboratory confirmed cases and 186 deaths (CFR, 3.51%) from 101 LGAs (18 States and FCT) during the same period in 2015 (Figure 8).
4.4. Cholera preparedness workshop held from 31st May – 1st June 2017 in Abuja to
develop Cholera preparedness plan as the season set in.
4.5. NCDC/partners provided onsite support in Kwara, Zamfara and Kebbi States.
4.6 NCDC/partners are providing onsite support in Borno State.
4.7. Preparedness and Response to Acute Watery Diarrhoea/ Cholera Guidelines have been finalised: http://ncdc.gov.ng/themes/common/docs/protocols/45_1507196550.pdf
4.8. States are enjoined to intensify surveillance, implement WASH activities and ensure early reporting.
5. CEREBROSPINAL MENINGITIS (CSM)
5.7. In the reporting week 46, 22 suspected Cerebrospinal Meningitis (CSM) cases were reported from 11 LGAs (eight States; Anambra – 1, Cross River – 6, Kaduna – 1, Katsina -2, Lagos – 1, Ondo – 1, Oyo – 2 & Sokoto - 8) compared with ten suspected cases from two LGAs (two States) at the same period in 2016
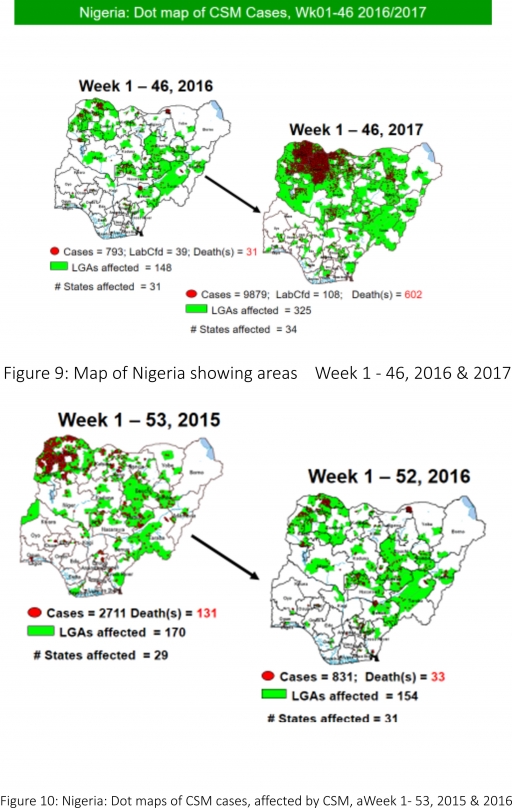
5.8. Between weeks 1 and 46 (2017), 9879 suspected CSM cases with 108 laboratory confirmed cases and 602 deaths (CFR, 6.09%) were recorded from 325 LGAs (34 States) compared with 793 suspected cases and 31 deaths (CFR, 3.91%) from 148 LGAs (31 States) during the same period in 2016 (Figure 9)
5.9. Between weeks 1 and 52, 2016, 831 suspected CSM cases with 43 laboratory confirmed cases and 33 deaths (CFR, 3.97%) were recorded from 154 LGAs (30 States and FCT) compared with 2,711 suspected cases and 131 deaths (CFR, 4.83%) from 170 LGAs (28 States and FCT) during the same period in 2015 (Figure 10)
5.10. Timeliness/completeness of CSM case-reporting from States to the National Level (2017 versus 2016): on average, 82.8% of the 26 endemic States sent CSM reports in a timely manner while 98.5% were complete in week 1 – 46, 2017 as against 85.8% timeliness and 99.2% completeness recorded within the same period in 2016
5.11. The National CSM Guidelines have been finalised and available via http://ncdc.gov.ng/themes/common/docs/protocols/51_1510449270.pdf
5.12. Enhanced surveillance/ case-based surveillance to begin 1st of December 2017, ahead of the 2017/2018 dry season
5.13. Development of State-specific CSM Epidemic Preparedness & Response plan completed in 11 Northern States within the Meningitis belt
5.14. Letters of alert have been developed and disseminated to all States with clear recommendations
6. GUINEA WORM DISEASE
6.7. In the reporting week, no rumour report of Guinea Worm disease was received from any State.
6.8. Nigeria has celebrated eight consecutive years of zero reporting of Guinea worm disease in the country. The Country has been officially certified free of Dracunculiasis transmission by the International Commission for the Certification of Dracunculiasis Eradication (ICCDE).
(For further information, contact Nigeria Guinea Worm Eradication Program / Neglected Tropical Diseases Division, Public Health Department/Federal Ministry of Health)

7. Update on national Influenza sentinel surveillance, Nigeria week 1 - 46, 2017
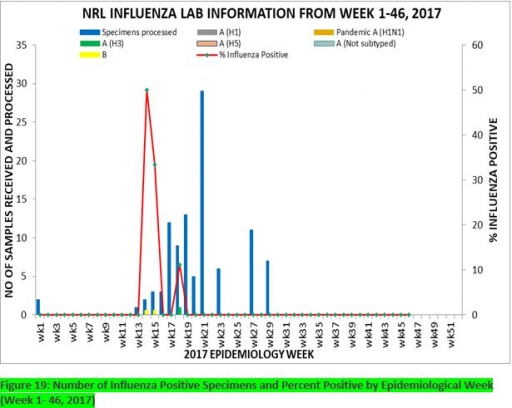
7.1. From week 1-46, a total of 107 suspected cases were reported, of which 99 were Influenza-like-illness (ILI), 8 Severe Acute Respiratory Infection (SARI).
7.2 A total of 107 samples were received and 103 samples were processed. Of the processed samples, 95(92.2%) were ILI cases, 8(7.8%) were Severe Acute Respiratory Infection (SARI).
7.4. Of the 95 processed ILI samples, 1(1.05%) was positive for Influenza A; 2(2.1%) positive for Influenza B and 92(98.95%) were negative.
7.5. Of the 8 processed SARI samples, none was positive for Influenza A and Influenza B.
7.6. 3(3.16%) of the processed 95 samples were positive for Influenza, with 1(33.3%) of these positive for Influenza A and 2(66.7%) positive for Influenza B.
7.7. The subtypes A seasonal H3, 2009A/H1N1 and A/not subtyped account for (100%), 0(0.0%) and 0(0.0%) of the total influenza A positive samples respectively.
7.8. The percentage influenza positive was highest (50.0%) in week 14, 2017
7.9. In the reporting week 46, four (4) samples were left unprocessed
FOR MORE INFORMATION CONTACT
Surveillance Unit:
Nigeria Centre for Disease Control,
801 Ebitu Ukiwe Street, Jabi, Abuja, Nigeria.
[email protected]
www.ncdc.gov.ng/reports
0800-970000-10










 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 