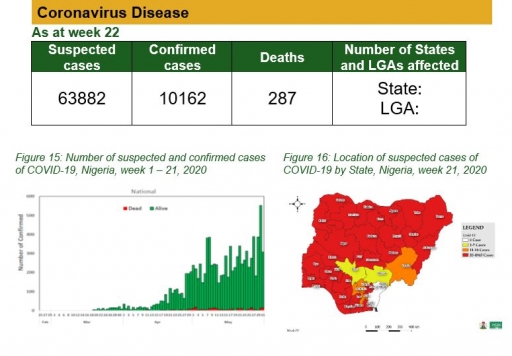
In the past few weeks, Nigeria has seen a significant increase in the number of COVID-19 cases reported across the country. As at the 31st of May 2020, there were 10,162 confirmed cases reported from 35 states and the Federal Capital Territory. The continuous and strategic expansion of COVID-19 testing centres across the country is one of the strategies that has enhanced the prompt detection of cases. To date, a total of 30 laboratories have been activated by the Nigeria Centre for Disease Control (NCDC) for the testing of COVID-19 in Nigeria.
This expansion has enhanced the turn-around-time from when samples are collected, to when results are communicated to individuals. However, an in-depth analysis of states’ sample collection shows that several states are not collecting adequate samples for testing including states with high positivity rates. The implication of this is the underutilisation of the national testing capacity and increased risk of asymptomatic cases going undetected and unintentionally spreading the virus.
To address this challenge, the national COVID-19 Emergency Operations Centre (EOC) convened a virtual meeting with all the states in the South-East as well as Borno, Jigawa and Katsina States. This was to discuss current sample collection strategies, challenges, and opportunities for improvement. One of the major challenges highlighted was inadequate operational funding for community surveillance, active case search and contact tracing.
1. Establish more sample collection centres possibly across all or strategically located Local Government Areas (LGAs) in their states
5. Ensure epi-number and other details of samples collected are entered into the Surveillance Outbreak Response Management and Analysis System (SORMAS) for accurate documentation
A major outcome of the meeting was for states to develop a plan of action to expand sample collection. The NCDC will continue to support states to effectively respond to this outbreak. However, we also encourage state governments to ensure that resources are adequately channeled towards addressing gaps in the COVID-19 response at the state level.
Summary of Incidents
Notes
1. Information for this disease was retrieved from the Technical Working Group and Situation Reports
2. Case Fatality Rate (CFR) for this disease is reported for confirmed cases only
3. Information for this disease was retrieved from IDSR 002 data
4. CFR for this disease is reported for total cases i.e. suspected + confirmed
5. Information for sentinel influenza was retrieved from the laboratory
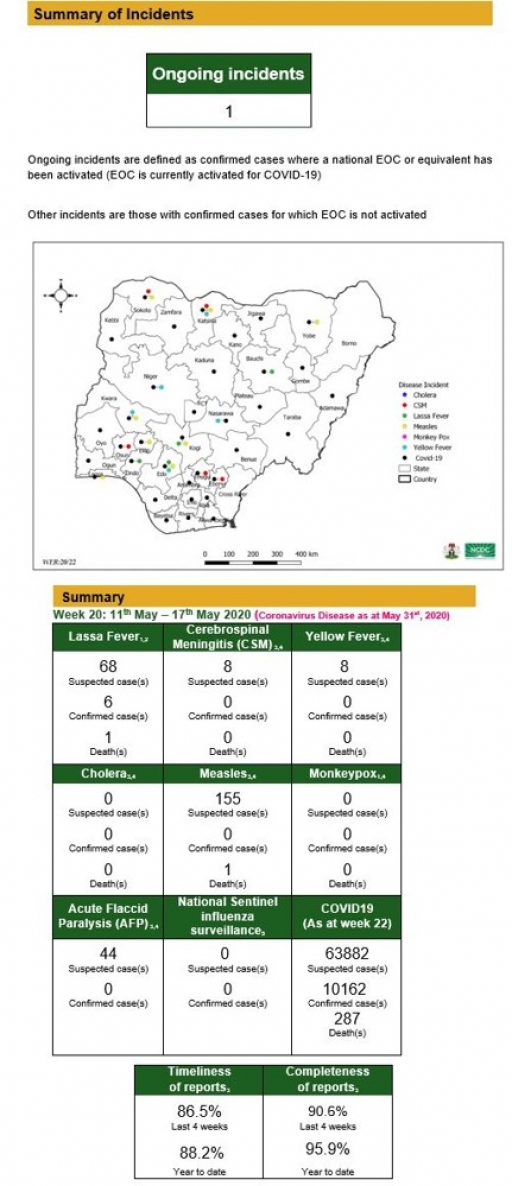
Lassa Fever
Key points
• There were 68 suspected cases, six confirmed cases and one death was recorded from five LGAs in three states
• No new healthcare worker was affected in the reporting week
Actions
To date:
• National rapid response and surge teams have been deployed from NCDC to support response activities in states
• State Public Health Emergency Operations Centre activated in affected States
• Developed Incident Action Plan to guide response activities
Planned:
• Resource mobilisation
• Pilot indigent patient treatment scheme through the basic healthcare provision funds
• Support states to develop and implement LF response sustainability plan
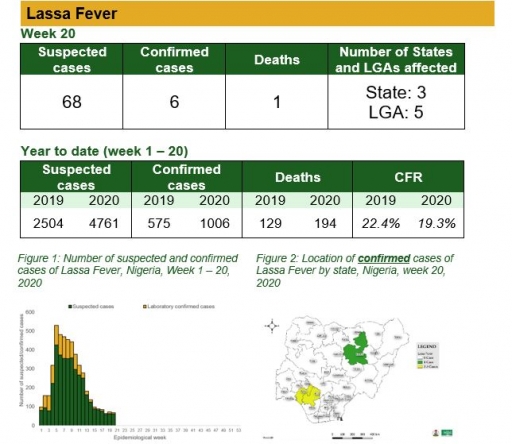
Cerebrospinal Meningitis (CSM)
Key points
There were eight suspected cases of Cerebrospinal Meningitis (CSM) reported from six LGAs in four states (Ebonyi – 3, Enugu – 1, Katsina – 3 & Sokoto – 1). None was laboratory confirmed and no death was recorded
Actions
To date:
• National CSM TWG meets weekly to review reports from states and plan appropriately
• Enhanced surveillance in all states
Planned:
• Continue harmonisation of the national line list and SORMAS data
• Continue to ensure that states reporting cases send their line lists and collect CSM samples
Yellow Fever
Key points
• There were eight suspected cases of Yellow Fever (YF) reported from seven LGAs in six states. None was laboratory confirmed and no death was recorded
Actions
To date:
• National multiagency YF Technical Working Group (TWG) is coordinating response activities
Planned:
• Surveillance and laboratory data harmonisation are ongoing

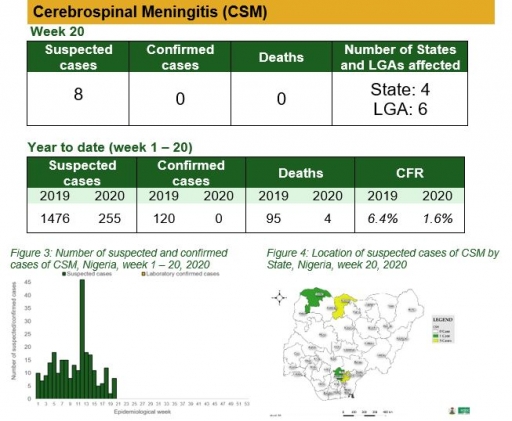
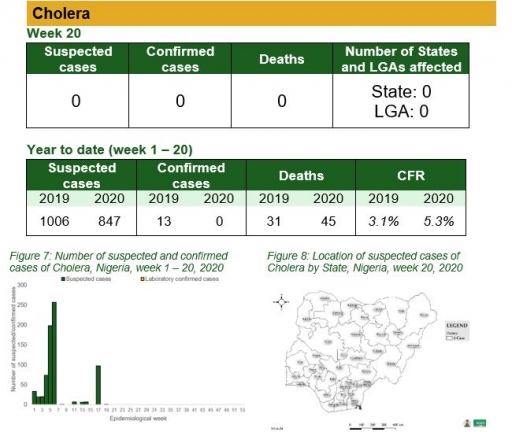
Cholera
Key points
• There was no case of cholera reported this week
Actions
To date
• National cholera multi-sectoral Technical Working Group (TWG) is monitoring all states and supporting already affected states
Planned:
• Continue follow up and monitoring of non-reporting states
• Continue harmonisation of the national line list and SORMAS data
Measles
Key points
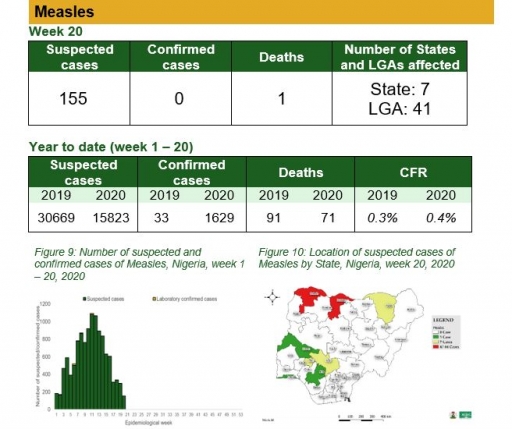
• There were 155 suspected cases of Measles reported from 41 LGAs in seven states. None was laboratory confirmed and one death was recorded
Actions
To date
• National Measles Technical Working Group (TWG) is closely monitoring surveillance data and response activities across the country
Planned:
• Intensify follow up with states to update and transmit line list
• Continue the review of measles surveillance data across the country
• Continue harmonisation of the national line list and SORMAS data
Monkeypox
Key points
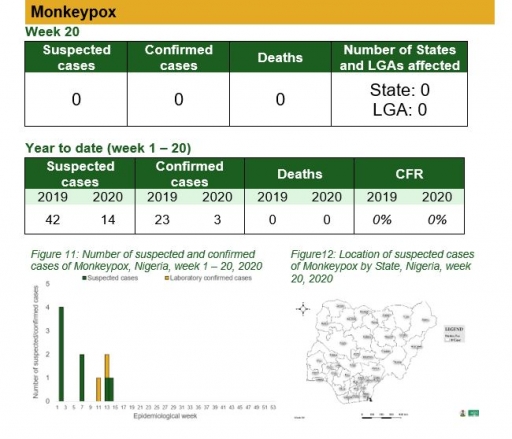
• There was no case of monkeypox reported this week
Actions
To date
• National Monkeypox Technical Working Group (TWG) is monitoring activities in all states
Planned:
• Enhance surveillance for monkeypox in high burden states
• Continue harmonisation of the national line list and SORMAS data
Acute Flaccid Paralysis (AFP)
Key points
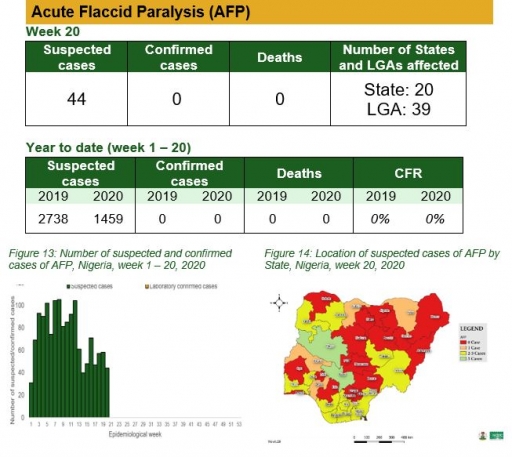
• There were 44 suspected cases of AFP reported from 39 LGAs in 20 states. None was laboratory confirmed and no death was recorded
Coronavirus (COVID-19)
Actions
To date:
• National COVID-19 multi-partner Emergency Operations Centre (EOC) continues to coordinate response activities across states.
• Accredited and activated thirty (30) testing laboratories across Nigeria so far
• Received and quarantined over 1200 returnees from other countries
• Printed and distributed the mandatory institutional quarantine guideline and NCDC CARE kit for the returnees and evacuees
• Operationalisation of next strategic directions following the COVID-19 National Outbreak Response Mid-action review meeting
• National Rapid Response Team continues to support affected states
• Collaborated with Africa CDC to deploy additional rapid response team to states
• Developed Home care interim guideline for COVID-19 patients which can be accessed via covid19.gov.ng
Planned:
• Work with states to scale up sample collection and testing
• Continue mobilisation of resources
• Continue to provide guidance to states in line with national guidelines and global best practices
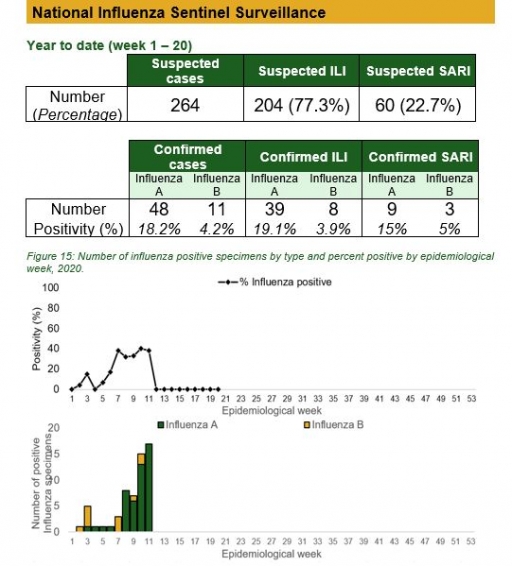
National Influenza Sentinel Surveillance
Key points
• The subtypes A seasonal H3, 2009A/H1N1 and A/not subtyped account for 0 (0.0%), 2 (9.5%) and 19 (90.5%) of the total influenza A positive sample respectively. The subtypes B VICTORIA, B Not subtyped and B Yamagata account for 0 (0.0%), 8 (100%) and 0 (0.0%) of the total influenza B positive samples respectively.
• The percentage influenza positive was highest in week 10 with 40%.
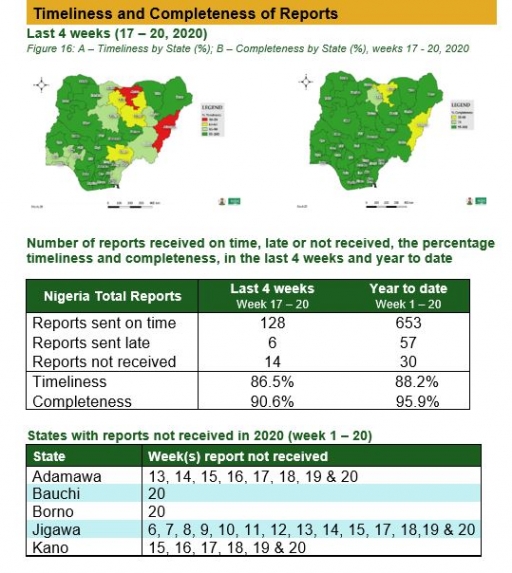
Timeliness and Completeness of Reports
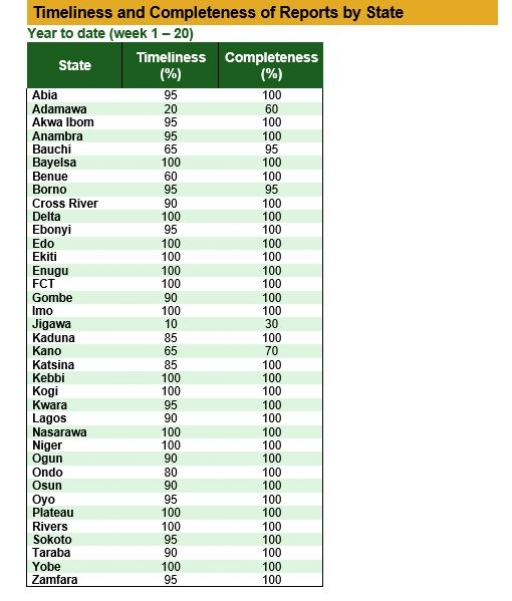
Timeliness and Completeness of Reports by State













 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 