Response to disease outbreaks in recent past has highlighted evidence of weakness in integration between disease surveillance and laboratories. In this week’s editorial, we review the roles of the laboratory before and during an outbreak.
1. Before an outbreak: The laboratory carries out two specific roles
• Early warning signals: This involves detecting pathogens that have the potential to spread. This will ensure early control measures are put in place.
• Outbreak Detection: The laboratory is responsible for confirming a diagnosis and providing guidance for a more specific case definition, detection of a new pathogen and providing additional information on the pathogen.
2. During an Outbreak
• Typing of the pathogen-this can help with linking clusters is situations when epidemiological data is insufficient
• Environmental investigations -This is applicable for disease conditions that are food and vector-borne e.g. Cholera, Yellow fever
3. In-between outbreaks
• Monitoring Endemic Disease Trends-This is achieved through confirmation of diagnosis (for case definitions that include laboratory criteria), monitoring resistance patterns of isolates and subtypes of a pathogen
• Monitoring eradication/elimination measures - Usually require more specific testing as positive predictive value decreases. Typing helps to identify the origin of a pathogen which will inform how eradication/elimination measures will be carried out.
It is very important that all States identify a public health laboratory – preferably in the State capital- to ensure laboratory confirmation of diseases, especially during outbreaks. Laboratory confirmation provides a stronger response structure to any outbreak.
Irrespective of the stage of the outbreak, it is important that good communication is established between the Epidemiologists and the laboratory. This also helps to increase on effective participation of the laboratory in surveillance
1. http://www.who.int/ihr/lyon/surveillance/lab_surveillance/en/
In the reporting week ending on December 10, 2017:
o There were 119 new cases of Acute Flaccid Paralysis (AFP) reported. None was confirmed as Polio. The last reported case of Polio in Nigeria was in August 2016. Active case search for AFP is being intensified as Nigeria has reinvigorated its efforts at eradicating Polio.
o 11 suspected cases of Cholera were reported from three LGAs in two States (Kaduna – 1 and Kano – 10). None was laboratory confirmed and no death was recorded.
o 18 suspected cases of Lassa fever were reported from six LGAs in four States (Bauchi – 1, Edo – 15, Ondo - 1 & Rivers - 1). One was laboratory confirmed and one death was recorded.
o There were 11 suspected cases of Cerebrospinal Meningitis (CSM) reported from nine LGAs in five States (Cross River – 1, Ebonyi – 1, Katsina -6, Osun – 1 & Oyo – 2). Of these, none was laboratory confirmed and one death was recorded. Ongoing surveillance for CSM has been intensified in all the 26 States in the Nigeria meningitis belt and case-based surveillance commenced from 4th December 2017.
o There were 238 suspected cases of Measles reported from 31 States. None was laboratory confirmed and two deaths were recorded.
In the reporting week, all States sent in their report. This is a remarkable improvement! Timeliness of reporting increases from 85% in week 48 to 86% in the current week (Week 49) while completeness remains at 100%. It is very important for all States to ensure timely and complete reporting at all times, especially during an outbreak.
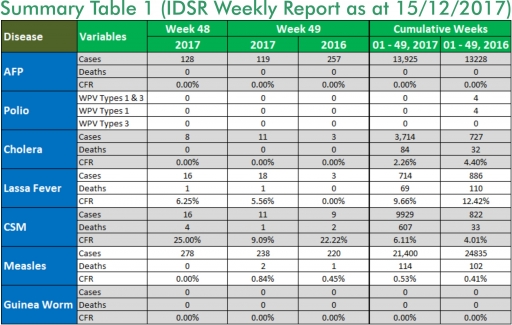
1. LASSA FEVER
Please note that the data reflects the routine reports i.e. all suspected cases including the laboratory positive and negative cases
1.1. 18 suspected cases of Lassa fever with one Laboratory confirmed and one death (CFR, 5.56%) were reported from six LGAs (four States: Bauchi – 1, Edo – 15, Ondo - 1 & Rivers - 1) in week 49, 2017 compared with three suspected cases reported from two LGAs (Bauchi State) at the same period in 2016
1.2. Laboratory results of the 18 suspected cases; one positive for Lassa fever (Ondo – 1), 17 were negative for Lassa fever & other VHFs (Bauchi – 1, Edo – 15 & Rivers - 1)
1.3. Between weeks 1 and 49 (2017), 714 suspected Lassa fever cases with 139 laboratory confirmed cases and 69 deaths (CFR, 9.66%) from 94 LGAs (28 States) were reported compared with 886 suspected cases with 93 laboratory confirmed cases and 110 deaths (CFR, 12.42%) from 141 LGAs (29 States) during the same period in 2016 (Figure 1)
1.4. Between weeks 1 and 52 2016, 921 suspected Lassa fever cases with 109 laboratory confirmed cases and 119 deaths (CFR, 12.92%) from 144 LGAs (28 States and FCT) were reported compared with 430 suspected cases with 25 laboratory confirmed cases and 40 deaths (CFR, 9.30%) from 37 LGAs (14 States and FCT) during the same period in 2015 (Figure 2)
1.5. Investigation and active case search ongoing in affected States with coordination of response activities by the NCDC with support from partners
1.5.1. National Lassa Fever Working Group meeting and weekly National Surveillance and Outbreak Response meeting on-going at NCDC to keep abreast of the current Lassa fever situation in the country
1.5.2. Response materials for VHFs provided to support States
1.5.3. New VHF guidelines have been developed by the NCDC (National Viral Haemorrhagic Fevers Preparedness guidelines, Infection Prevention and Control of VHF and Standard Operating Procedures for Lassa fever management) and are available on the NCDC website- http://ncdc.gov.ng/diseases/guidelines
1.5.4. VHF case-based forms completed by affected States are being entered into the new VHF management system. This system allows for the creation of a VHF database for the country. Data from the VHF database is currently being analysed to inform decision making in the coming year
1.5.5. Confirmed cases are being treated at identified treatment/isolation centres across the States with Ribavirin and necessary supportive management also instituted
1.5.6. Onsite support was earlier provided to Ogun, Nasarawa, Taraba, Ondo and Borno States by the NCDC and partners
1.5.7. Offsite support provided by NCDC/partners in all affected States
1.5.8. States are enjoined to intensify surveillance and promote Infection, Prevention and Control (IPC) measures in health facilities
1.5.9. Ongoing visits to support priority States in developing preparedness and response plans ahead of dry season
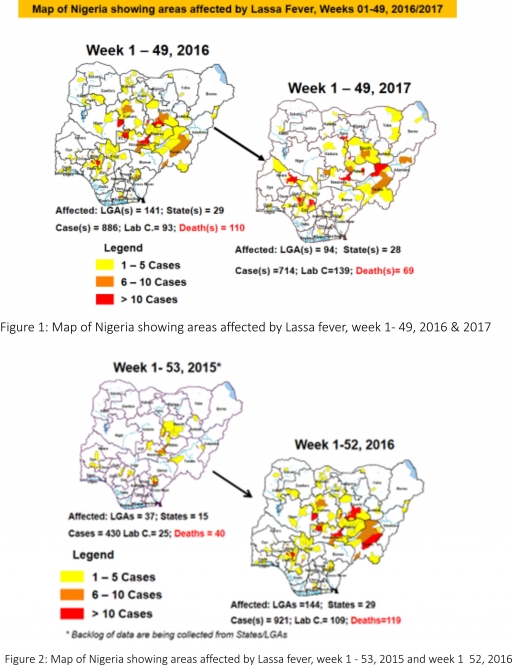
2. MEASLES
2.1. In the reporting week, 238 suspected cases of Measles and two deaths (CFR, 0.84%) were reported from 31 States compared with 220 suspected cases with two Laboratory confirmed cases and one death (CFR, 0.41%) reported from 32 States during the same period in 2016
2.2. So far, 21,400 suspected Measles cases with 109 laboratory confirmed cases and 114 deaths (CFR, 0. 53%) have been reported in 2017 from 36 States and FCT (Figure 4) compared with 24,835 suspected cases and 102 deaths (CFR, 0.41%) from 36 States and FCT during the same period in 2016
2.3. In 2016 (week 1 -52), 25,251 suspected Measles cases with 102 deaths (CFR, 0.40%) were reported from 36 States and FCT compared with 24,421 suspected cases with 127 deaths (CFR, 0.52%) during the same period in 2015 (Figure 5)
2.4. Response measures include immunization for all vaccine-preventable diseases in some selected/affected wards/LGAs during SIAs, as well as case management
2.5. Scheduled Measles campaigns in the North East were conducted from 12th – 17th January 2017 in Adamawa, Borno and Yobe States (Phase I) and Phase II from 21st – 25th January 2017 in Borno State and 4th – 8th February 2017 in Yobe State
2.6. Measles Surveillance Evaluation and Establishment of the burden of Congenital Rubella Syndrome (CRS) in 12 selected States in the six geopolitical zones from the 17th -21st July 2017 conducted
2.7. Measles mass campaign conducted in seven North West and North East States from 9th – 14th November 2017 and 30th November – 5th December 2017 respectively.
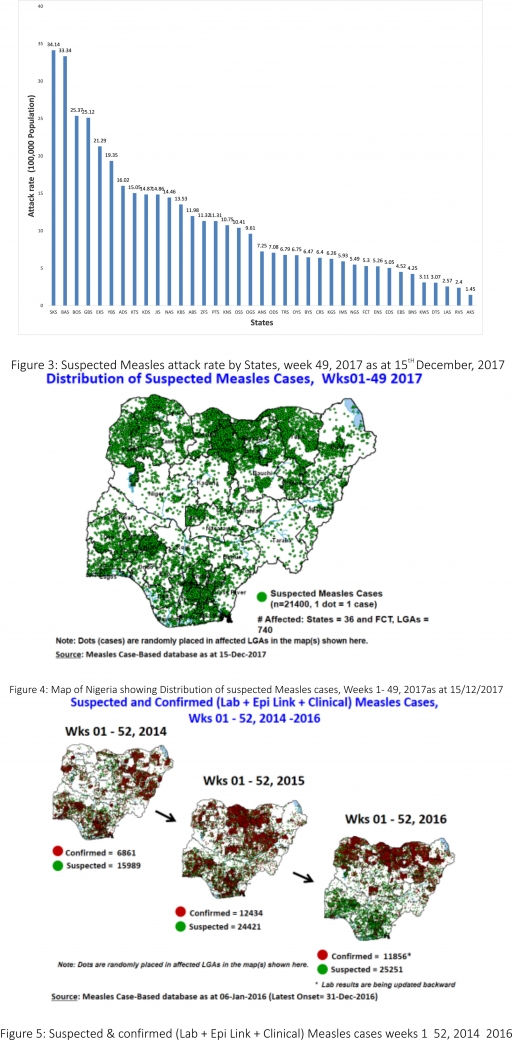
3. POLIOMYELITIS
3.1. As at December 8th, 2017, no new case of WPV was recorded
3.2. Three new cVDPV2, environmental derived and Polio compatible cases identified
3.2.1. In the reporting week, 119 cases of AFP were reported from 104 LGAs in 31 States and FCT
3.2.2. AFP Surveillance has been enhanced and outbreak response is on-going in Borno and other high-risk States
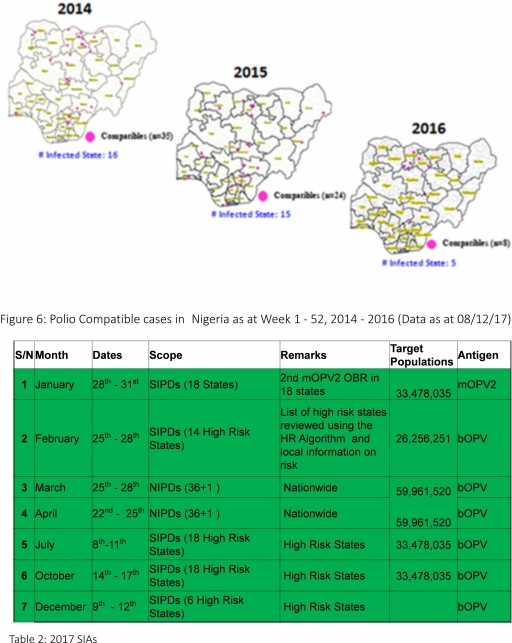
3.2.3. The 1st round of SIPDs in 2017 was conducted from 28th – 31st January 2017 in the 18 high-risk States. This was carried out using mOPV2 (2nd mOPV2 OBR). The schedule for other SIAs is as described in Table 2
3.2.4. The 2nd and 3rd round of SIPDs completed (25th-28th February and 8th – 11th July 2017) in 14 & 18 high-risk States using bOPV respectively.
3.2.5. The 1st and 2nd rounds of NIPDs completed (from 25th – 28th March 2017 and 22nd – 25th April 2017) nationwide respectively.
3.2.6. The 4th round of SIPDs completed from 14th- 17th October 2017 in 18 high-risk States using bOPV.
3.2.7. The 5th round of SIPDs completed from 9th- 12th December 2017 in 6 high-risk States using bOPV.
3.2.8. Between weeks 1 and 52 in 2016, four WPVs were isolated from Borno State compared to no WPV isolated during the same period in 2015.
3.3. No circulating Vaccine Derived Polio Virus type 2 (cVDPV2) was isolated in week 1 - 52, in both 2016 and 2015.
3.4. Between weeks 1 and 52, 2016 two (2) cVDPV2 were isolated in two LGAs (two States) while one (1) cVDPV2 was isolated from Kwali, FCT during the same period in 2015.
3.5. Six confirmed WPVs were isolated in 2014.
3.6. The SIAs were strengthened with the following events:
3.6.1. Immunisation for all vaccine-preventable diseases in some selected wards/LGAs.
3.6.2. Use of health camp facilities.
3.6.3. Field supportive supervision and monitoring.
3.6.4. Improved Enhanced Independent Monitoring (EIM) and Lots Quality Assessments (LQAs) in all Polio high-risk States.
3.6.5. High level of accountability framework
4. CHOLERA
4.1. 11 suspected cases of Cholera were reported from three LGAs (two States; Kano – 10 & Kaduna -1) in week 49 compared with three suspected cases reported from Igabi LGA (Kaduna State) during the same period in 2016.
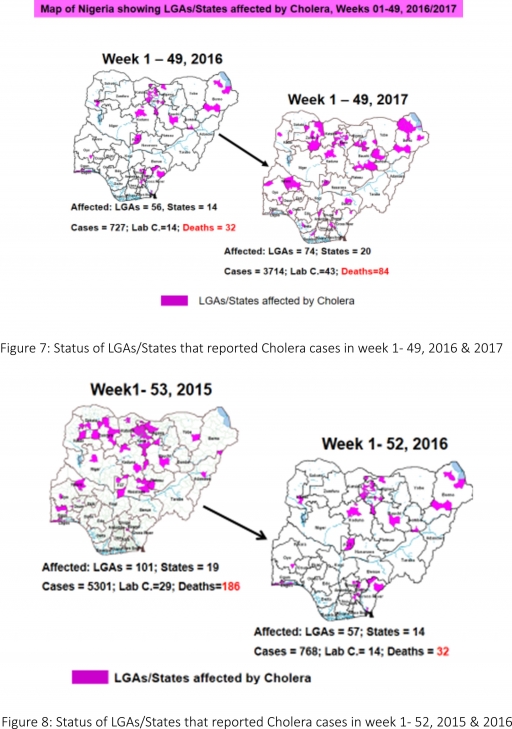
4.2. Between weeks 1 and 49 (2017), 3714 suspected Cholera cases with 43 laboratory confirmed and 84 deaths (CFR, 2.26%) from 74 LGAs (20 States) were reported compared with 727 suspected cases and 32 deaths (CFR, 4.40%) from 56 LGAs (14 States) during the same period in 2016 (Figure 7).
4.3. Between weeks 1 and 52 (2016), 768 suspected Cholera cases with 14 laboratory confirmed cases and 32 deaths (CFR, 4.17%) from 57 LGAs (14 States) were reported compared with 5,301 cases with 29 laboratory confirmed cases and 186 deaths (CFR, 3.51%) from 101 LGAs (18 States and FCT) during the same period in 2015 (Figure 8).
4.4. Cholera preparedness workshop held from 31st May – 1st June, 2017 in Abuja to
develop Cholera preparedness plan as the season set in.
4.5. NCDC/partners provided onsite support in Kwara, Zamfara and Kebbi States.
4.6 NCDC/partners are providing onsite support in Borno State.
4.7. Preparedness and Response to Acute Watery Diarrhoea/ Cholera Guidelines have been finalised: http://ncdc.gov.ng/themes/common/docs/protocols/45_1507196550.pdf
4.8. States are enjoined to intensify surveillance, implement WASH activities and ensure early reporting.
5. CEREBROSPINAL MENINGITIS (CSM)
5.1 In the reporting week 49, 11 suspected Cerebrospinal Meningitis (CSM) cases and one death (CFR, 9.09%) were reported from nine LGAs (five States; Cross River – 1, Ebonyi – 1, Katsina - 6, Osun – 1 & Oyo – 2) compared with nine suspected cases and two deaths (CFR,22.22%) from four LGAs (four States) at the same period in 2016
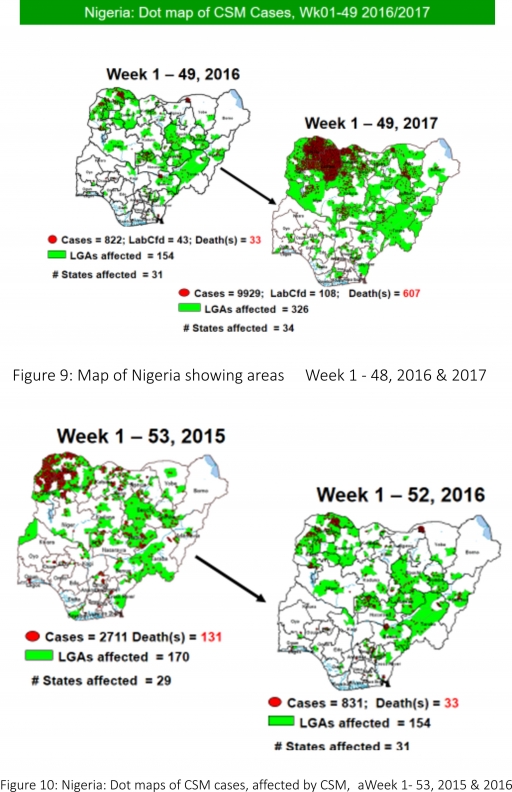
5.2 Between weeks 1 and 48 (2017), 9929 suspected CSM cases with 108 laboratory confirmed cases and 607 deaths (CFR, 6.11%) were recorded from 326 LGAs (34 States) compared with 822 suspected cases and 33 deaths (CFR, 4.01%) from 154 LGAs (31 States) during the same period in 2016 (Figure 9)
5.3 Between weeks 1 and 52, 2016, 831 suspected CSM cases with 43 laboratory confirmed cases and 33 deaths (CFR, 3.97%) were recorded from 154 LGAs (30 States and FCT) compared with 2,711 suspected cases and 131 deaths (CFR, 4.83%) from 170 LGAs (28 States and FCT) during the same period in 2015 (Figure 10)
5.4 Timeliness/completeness of CSM case-reporting from States to the National Level (2017 versus 2016): on average, 83.1% of the 26 endemic States sent CSM reports in a timely manner while 99.3% were complete in week 1 – 49, 2017 as against 85.5% timeliness and 98.7% completeness recorded within the same period in 2016
5.5 The National CSM Guidelines have been finalised and available via http://ncdc.gov.ng/themes/common/docs/protocols/51_1510449270.pdf
5.6 Enhanced surveillance/ case-based surveillance to begin 1st of December 2017, ahead of the 2017/2018 dry season
5.7 Development of State-specific CSM Epidemic Preparedness & Response plan completed in 11 Northern States within the Meningitis belt
5.8 Letters of alert have been developed and disseminated to all States with clear recommendations
5.9 The National CSM Emergency Operations Centre has been activated and is currently in alert mode
6 GUINEA WORM DISEASE
6.1 In the reporting week, no rumour report of Guinea Worm disease was received from any State.
6.2 Nigeria has celebrated eight consecutive years of zero reporting of Guinea worm disease in the country. The Country has been officially certified free of Dracunculiasis transmission by the International Commission for the Certification of Dracunculiasis Eradication (ICCDE).
(For further information, contact Nigeria Guinea Worm Eradication Program / Neglected Tropical Diseases Division, Public Health Department/Federal Ministry of Health)

7. Update on national Influenza sentinel surveillance, Nigeria week 1 - 50, 2017
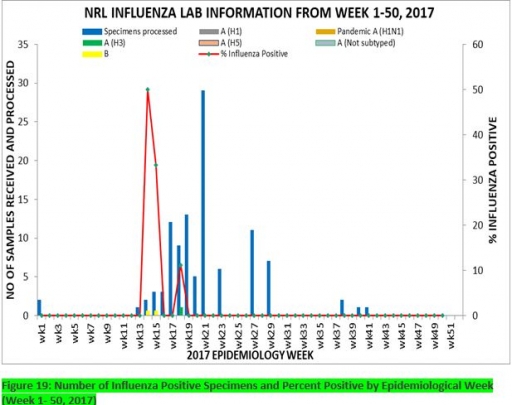
7.1. From week 1-50, a total of 107 suspected cases were reported, of which 99 were Influenza like-illness (ILI), 8 Severe Acute Respiratory Infection (SARI).
7.2 A total of 107 samples were received and 107 samples were processed. Of the processed samples, 99(92.5%) were ILI cases, 8(7.5%) were Severe Acute Respiratory Infection (SARI).
7.4. Of the 99 processed ILI samples, 1(1.01%) was positive for Influenza A; 2(2.02%) positive for Influenza B and 96(96.97%) were negative.
7.5. Of the 8 processed SARI samples, none was positive for Influenza A and Influenza B.
7.6. 3(2.80%) of the processed 107 samples were positive for Influenza, with 1(33.3%) of these positive for Influenza A and 2(66.7%) positive for Influenza B.
7.7. The subtypes A seasonal H3, 2009A/H1N1 and A/not subtyped account for (100%), 0(0.0%) and 0(0.0%) of the total influenza A positive samples respectively.
7.8. The percentage influenza positive was highest (50.0%) in week 14, 2017
7.9. In the reporting week 50, none samples were left unprocessed
FOR MORE INFORMATION CONTACT
Surveillance Unit:
Nigeria Centre for Disease Control,
801 Ebitu Ukiwe Street, Jabi, Abuja, Nigeria.
[email protected]
www.ncdc.gov.ng/reports
0800-970000-10










 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 