With the resumption of international flights, the Federal Government of Nigeria through the Presidential Task Force on COVID-19 (PTF-COVID-19) has continued to put in place public health measures to prevent further spread of the virus in Nigeria. One of such is the establishment of guiding protocols and procedures for all return travellers using the mandatory quarantine protocol.
In line with this, the Nigeria International Travel Portal (NITP) was recently launched by PTF-COVID-19 with the support of the Coalition Against COVID-19 (CACOVID).This is to ensure that all intending travellers are adequately equipped with necessary information regarding COVID-19 preventive measures, ensure safe travel and reduce the risk of a spike in COVID-19 cases in Nigeria. The NITP is hosted on the web-portal of the Nigeria Centre for Disease Control (NCDC) and co-managed with the Port Health Services of the Federal Ministry of Health, PTF-COVID-19 and other relevant government institutions.
Every air traveller is expected to be familiar with the required protocol before and upon arrival in Nigeria. Below are some of the key information every traveller must be acquainted with:
1. Before your trip to Nigeria, kindly visit htttp://ntip.ncdc.gov.ng to fill out your pre-boarding and health declaration screening form
2. Upload your negative PCR test results taken not more than 96 hours before departure
3. Select an appointment date and private laboratory of preference for a repeat COVID-19 test to be done within 7 days of arrival in Nigeria. Obtain barcode after payment has been made OR make payment upon arrival in Nigeria
4. Self-isolate on arrival in Nigeria and take COVID-19 test 7 days later on the allocated appointment date
5. All travel-related testing must be carried out in accredited private laboratories in Nigeria. You can access list of these laboratories via https://covid19.ncdc.gov.ng/privatelabs/.
All payments received on the platform are sent directly to the testing laboratories where passengers have chosen to be tested. For passengers that may encounter challenges with the travel portal, kindly see the NCDC frequently asked questions on https://covid19.ncdc.gov.ng/nitpfaq/.
Summary of Incidents
Notes
1. Information for this disease was retrieved from the Technical Working Group and Situation Reports
2. Case Fatality Rate (CFR) for this disease is reported for confirmed cases only
3. Information for this disease was retrieved from IDSR 002 data
4. CFR for this disease is reported for total cases i.e. suspected + confirmed
5. Information for sentinel influenza was retrieved from the laboratory
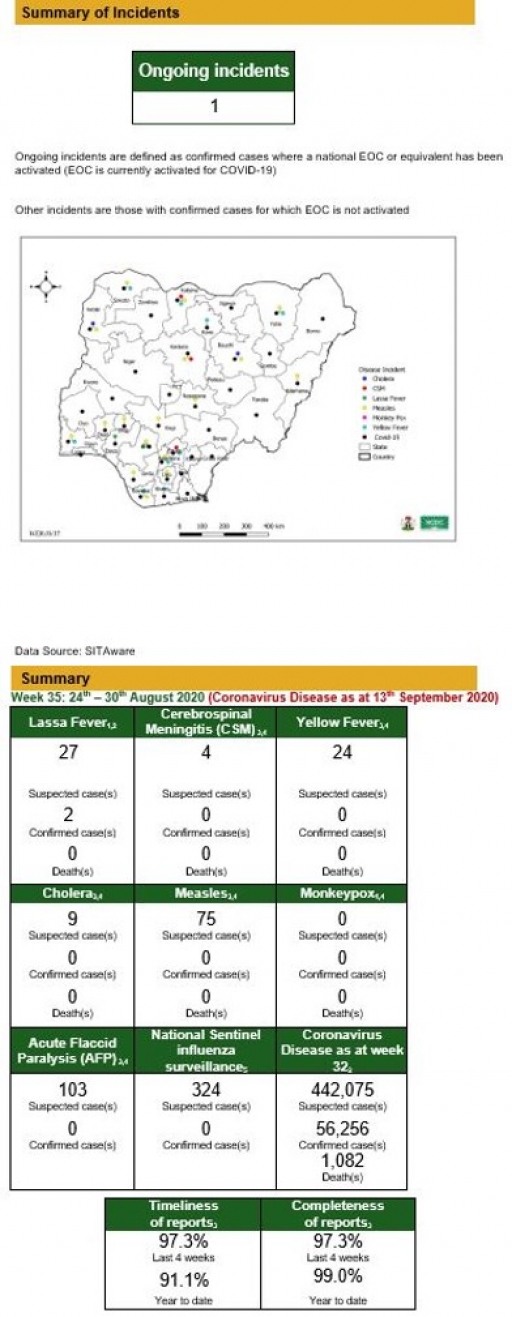
Lassa Fever
Key points
• There were 27 suspected cases, two were laboratory confirmed and no death was recorded from two LGAs in two states.
• No healthcare worker was infected in the reporting week
Actions
To date:
• National Lassa fever multi-partner, multi-sectoral Technical Working Group (TWG) continues to coordinate the response activities at all levels
• Enhanced surveillance (contact tracing and active case finding) ongoing in affected states
Planned:
• Continue mobilisation of resources
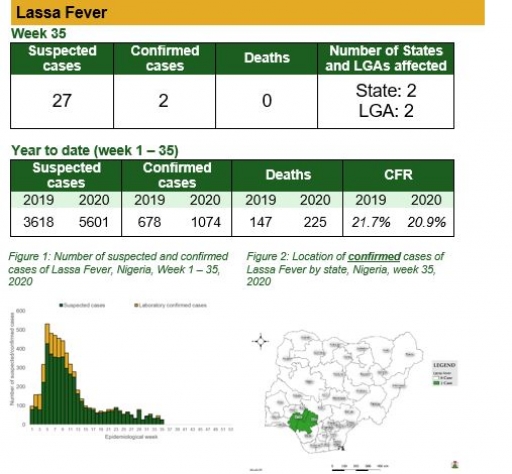
Cerebrospinal Meningitis (CSM)
Key points
There were four suspected cases of Cerebrospinal Meningitis (CSM) reported from four LGAs in three states (Enugu – 1, Kaduna – 2 & Katsina – 1). There was no laboratory confirmed case and no death was recorded
Actions
To date:
• National CSM TWG meets weekly to review reports from states and plan appropriately
• Enhanced surveillance in all states
Planned:
• Continue harmonisation of the national line list and SORMAS data
• Continue to ensure that states reporting cases send their line lists and collect CSM samples
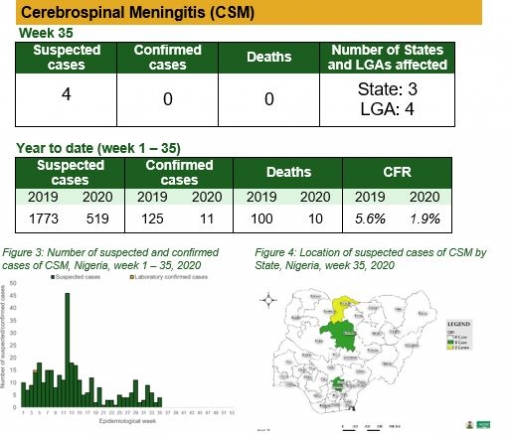
Yellow Fever
Key points
• There were 24 suspected cases of Yellow Fever (YF) reported from 20 LGAs in 12 states. None was laboratory confirmed and no death was recorded
Actions
To date:
• National multiagency YF Technical Working Group (TWG) is coordinating response activities
Planned:
• Continue harmonisation of surveillance and laboratory data ongoing
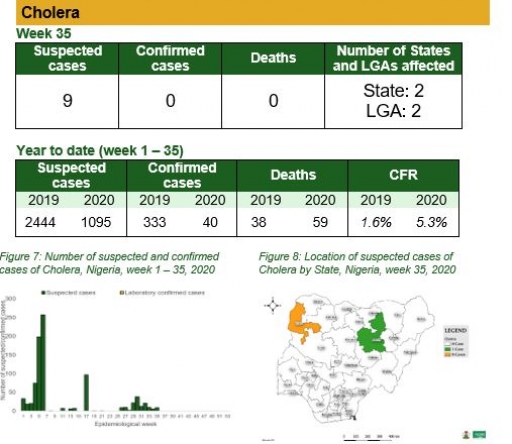
Cholera
Key points
• There were nine suspected cases of cholera reported from two LGAs in two states (Bauchi – 1 & Kebbi – 8). None was laboratory confirmed and no death was recorded
Actions
To date
• National Cholera Multi-Sectoral Technical Working Group (TWG) is monitoring all states and supporting affected states
Planned:
• Continue follow up and monitoring of non-reporting states
• Continue harmonisation of the national line list and SORMAS data
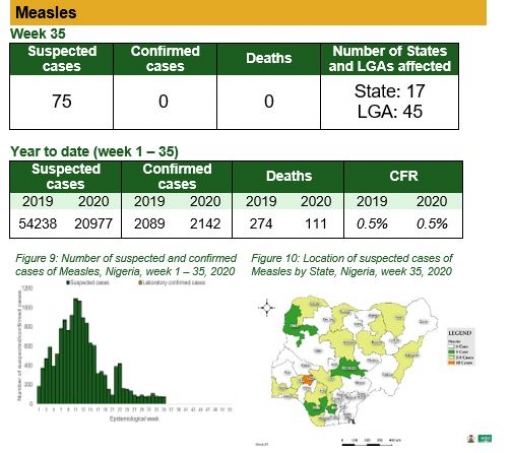
Measles
Key points
• There were 75 suspected cases of measles reported from 45 LGAs in 17 states. None was laboratory confirmed and no death was recorded
Actions
To date
• National Measles TWG is closely monitoring measles surveillance data and providing feedback to relevant agencies and development partners
• Weekly surveillance and laboratory data harmonisation ongoing
Planned:
• Intensify follow up with states to update and transmit line list
• Continue monthly measles surveillance data review
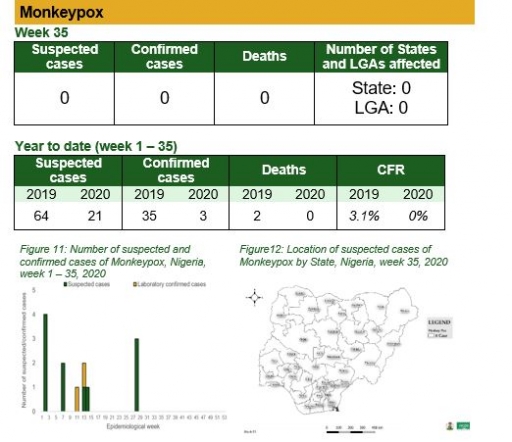
Monkeypox
Key points
• There was no suspected case of Monkeypox reported this week
Actions
To date
• National Monkeypox Technical Working Group (TWG) is monitoring activities in all states
Planned:
• Enhance surveillance for monkeypox in high burden states
• Continue harmonisation of the national line list and SORMAS data
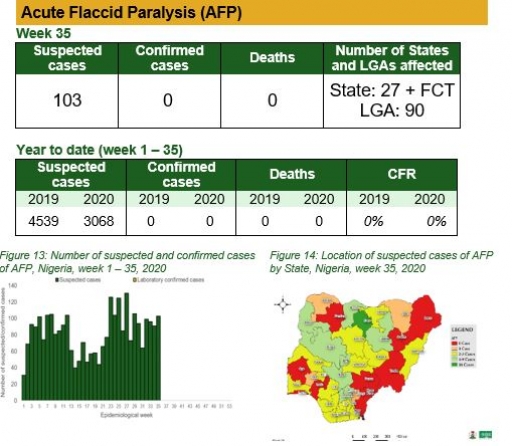
Acute Flaccid Paralysis (AFP)
Key points
• There were 103 suspected cases of AFP reported from 90 LGAs in 27 states and FCT. None was laboratory confirmed and no death was recorded
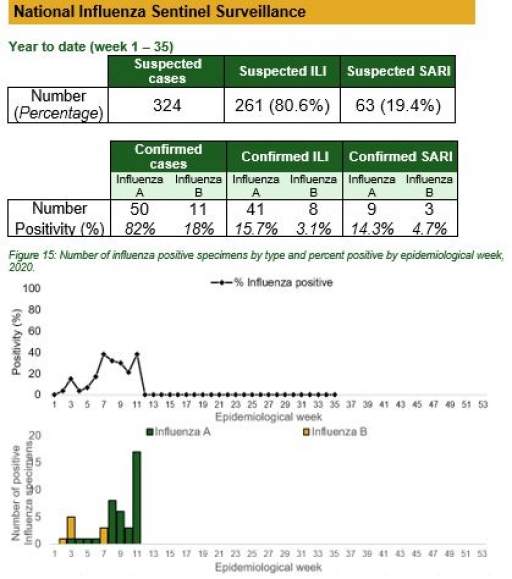
National Influenza Sentinel Surveillance
Key points
• The subtypes A seasonal H3, 2009A/H1N1 and A/not subtyped account for 0(0.0%), 17(34.0%) and 33(66.0%) of the total influenza A positive sample respectively. The subtypes B VICTORIA, B Not subtyped and B Yamagata account for 3(27.3.3%), 8(72.7%) and 0(0.0%) of the total influenza B positive samples respectively.
• The percentage influenza positive was highest in week 10 with 39.5%.
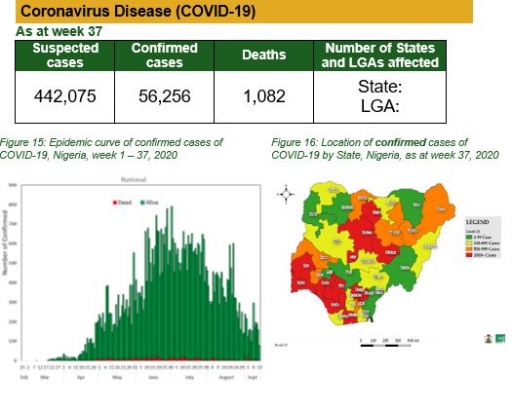
Coronavirus Disease (COVID-19)
Actions
To date:
• National COVID-19 multi-partner Emergency Operations Centre (EOC) continues to coordinate response activities across states
• Commenced auditing of the Data Quality Implementation platform (DQIP) in states
• Technical advisors deployed to states to support implementation of REDISSE COVID-19 projects
• Feedback provided to State IPC focal persons and partners
• Conducted Risk Communication training of correctional facilities staff on how to deliver key messages using physical distancing techniques
Planned:
• Planning engagement meeting with religious leaders
• Conduct training on sample collection, packaging and transportation
• Finalise the draft guidelines on intensified surveillance in 'silent LGAs' across the country (LGAs that have not reported a single suspected case)
• Monitoring of states implementation of surveillance activities in the IAP
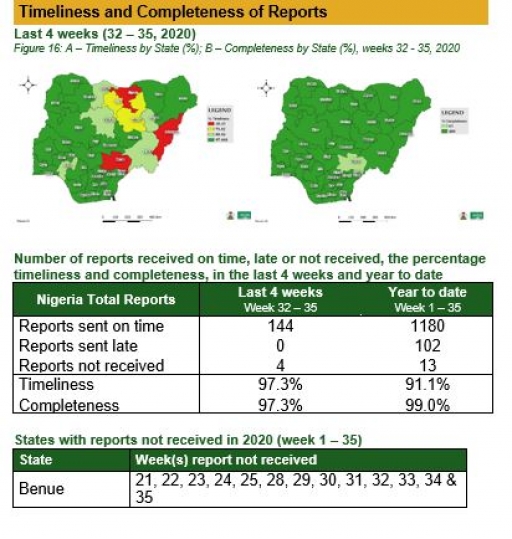
Timeliness and Completeness of Reports
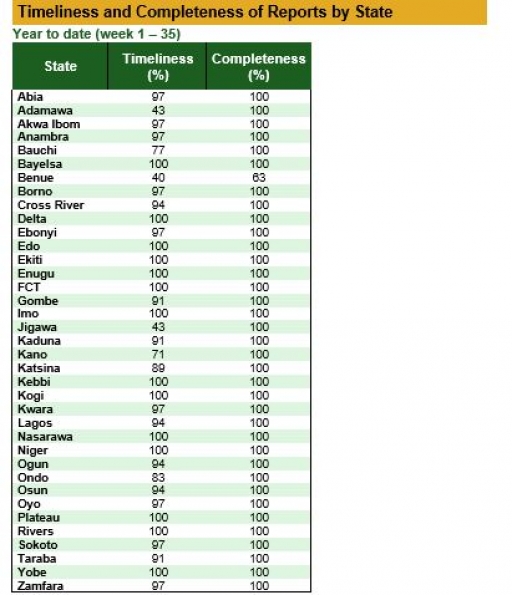
Timeliness and Completeness of Reports by State














 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 