Editoral
NCDC Publishes an Updated National COVID-19 Case Management Guidelines
Posted: 09-06-2020 04:15:20 PM
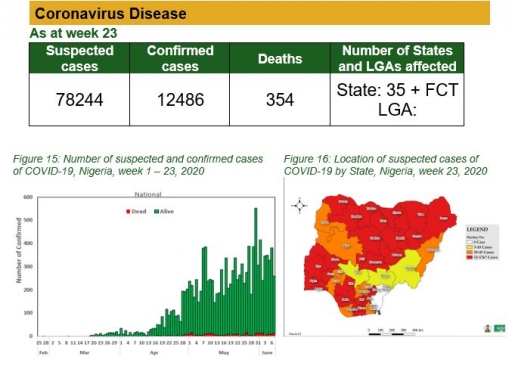
Nigeria is one of several countries affected by the coronavirus disease (COVID-19) pandemic. As at the 8th of June, 2020, a total of 12,801 cases and 361 deaths have been recorded across 35 states and the Federal Capital Territory. As part of its mandate, the Nigeria Centre for Disease Control (NCDC) has continued to provide evidence-based guidelines on the management of COVID-19 cases in Nigeria.
Based on the World Health Organization’s new guidelines on case management, released on the 27th of May 2020, the NCDC has also published a third version of the National Interim Guideline for Clinical Case Management of COVID-19. In line with evolving knowledge of the disease, this revision has been done within the Nigerian context. The aim of this is to standardise care, significantly improve treatment outcomes for confirmed cases and foster understanding of clinical management pathway for COVID-19. Below are few key extracts from the revised guidelines:
1. A suspect case is defined as any person (including severely ill patients) presenting with fever, cough or difficulty in breathing AND who within 14 days before the onset of illness had travel history to any country with confirmed and ongoing community transmission of SARS-CoV-2 OR close contact with a confirmed case of COVID-19 OR exposure to a healthcare facility where COVID-19 case(s) have been reported
2. A confirmed case person is one with laboratory confirmation of SARS-CoV-2 infection with or without signs and symptoms
3. A COVID-19 death is defined as a death resulting from a clinically compatible illness, in a probable or confirmed COVID-19 case, unless there is a clear alternative cause of death that cannot be related to COVID-19 disease (e.g. trauma).
4. For samples, minimum of one (1) nasal swab and one (1) oropharyngeal swab should be collected. Sputum should be collected from patients with a productive cough
5. For mild cases, management includes antipyretics; rest; and close monitoring of all vital signs. Multivitamins and oral rehydration and mineral supplements may be administered but prophylactic antibiotics should NOT be given to asymptomatic or mildly symptomatic patients
6. For severe cases, provision of supplemental oxygen therapy is a hallmark of treatment. There should be single use oxygen delivery interfaces (nasal cannula, simple face mask, and mask with reservoir bag)
7. As there is no evidence of their efficacy, the following drugs should not be administered except during clinical trials: Chloroquine and hydroxychloroquine (+/- azithromycin) and antivirals, including but not limited to Lopinavir/ritonavir, Remdesivir, Umifenovir and Favipiravi
8. Evidence has revealed that “viral RNA detected beyond 10 days is no longer infectious as no viable virus is grown by viral culture.†Hence, the following discharge criteria apply:
a. For symptomatic cases: Ten (10) days after symptom onset, plus at least 3 days without symptoms (fever and respiratory symptoms) as well as SpO2 ≥ 95% in room air for 3 days
b. For asymptomatic cases: Fourteen (14) days after the initial positive result (date of collection of sample)
9. Discharged patients should continue self-isolation one week after discharge home (see self-isolation guideline here). Patients should be followed up the first week of discharge and monthly for the next three months.
10. Home care is reserved for clinically stable cases with no co-morbidity (for those >50 years) nor communicable disease (for those
Summary of Incidents
Notes
1. Information for this disease was retrieved from the Technical Working Group and Situation Reports
2. Case Fatality Rate (CFR) for this disease is reported for confirmed cases only
3. Information for this disease was retrieved from IDSR 002 data
4. CFR for this disease is reported for total cases i.e. suspected + confirmed
5. Information for sentinel influenza was retrieved from the laboratory
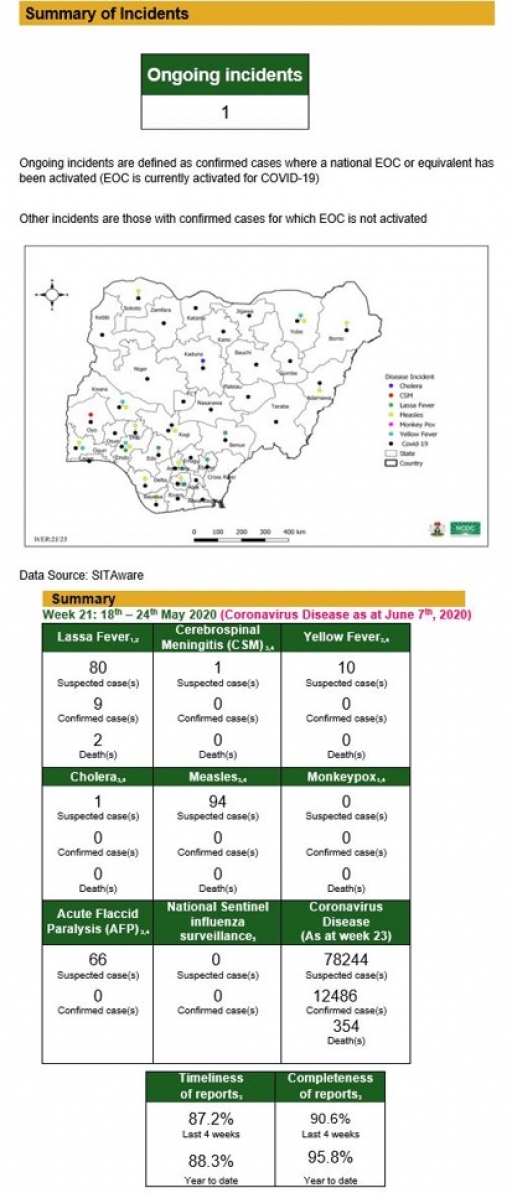
Lassa Fever
Key points
• There were 80 suspected cases, nine confirmed cases and two deaths were recorded from 37 LGAs in ten states
• One new healthcare worker was affected in the reporting week
Actions
To date:
• National Lassa fever (LF) multi-partner, multi-sectoral Technical Working Group (TWG) continues to coordinate the response activities at all levels
• State Public Health Emergency Operations Centre activated in affected States
• Developed Incident Action Plan to guide response activities
Planned:
• Resource mobilisation
• IPC training for healthcare workers
• Finalisation of the LF five-year strategic plan
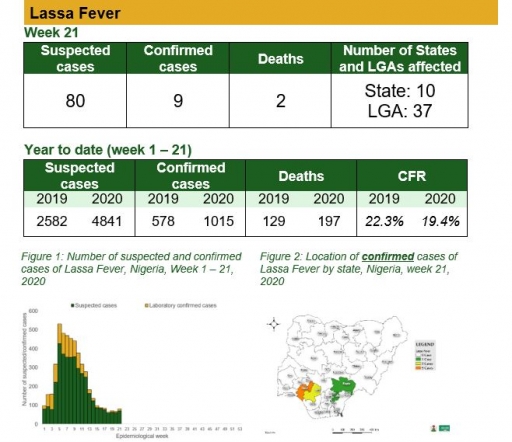
Cerebrospinal Meningitis (CSM)
Key points
There was one suspected case of Cerebrospinal Meningitis (CSM) reported from one LGA in Oyo state. None was laboratory confirmed and no death was recorded
Actions
To date:
• National CSM TWG meets weekly to review reports from states and plan appropriately
• Enhanced surveillance in all states
Planned:
• Continue harmonisation of the national line list and SORMAS data
• Continue to ensure that states reporting cases send their line lists and collect CSM samples
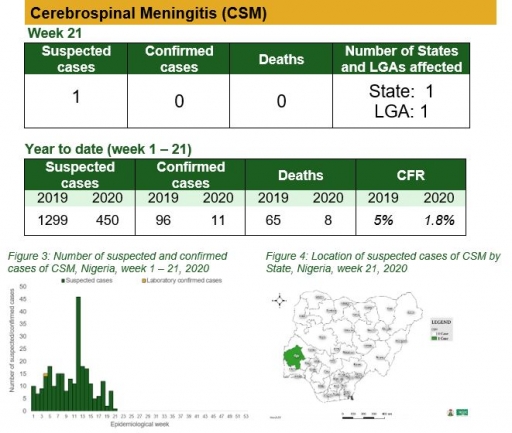
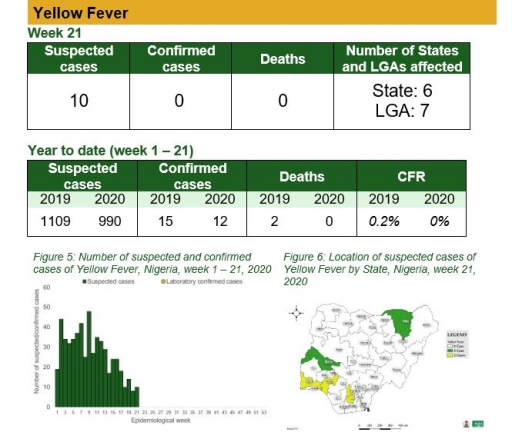
Yellow Fever
Key points
• There were ten suspected cases of Yellow Fever (YF) reported from seven LGAs in six states. None was laboratory confirmed and no death was recorded
Actions
To date:
• National multiagency YF Technical Working Group (TWG) is coordinating response activities
Planned:
• Surveillance and laboratory data harmonisation are ongoing
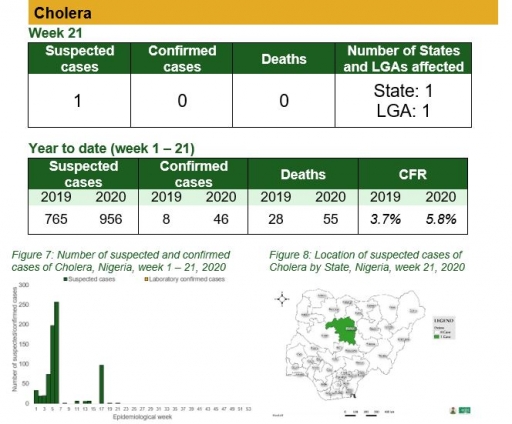
Cholera
Key points
• There was one case of cholera reported in one LGA in Kaduna state. None was laboratory confirmed and no death was recorded
Actions
To date
• National cholera multi-sectoral Technical Working Group (TWG) is monitoring all states and supporting already affected states
Planned:
• Continue follow up and monitoring of non-reporting states
• Continue harmonisation of the national line list and SORMAS data
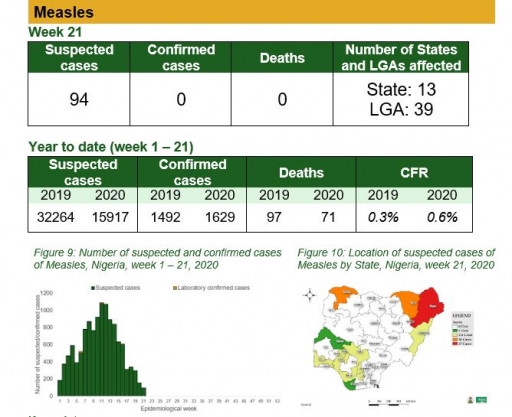
Measles
Key points
• There were 94 suspected cases of measles reported from 39 LGAs in 13 states. None was laboratory confirmed and no death was recorded
Actions
To date
• National Measles Technical Working Group (TWG) is closely monitoring surveillance data and response activities across the country
Planned:
• Intensify follow up with states to update and transmit line list
• Continue the review of measles surveillance data across the country
• Continue harmonisation of the national line list and SORMAS data
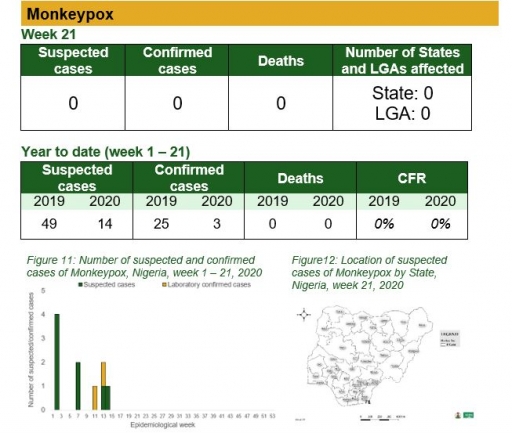
Monkeypox
Key points
• There was no case of monkeypox reported this week
Actions
To date
• National Monkeypox Technical Working Group (TWG) is monitoring activities in all states
Planned:
• Enhance surveillance for monkeypox in high burden states
• Continue harmonisation of the national line list and SORMAS data
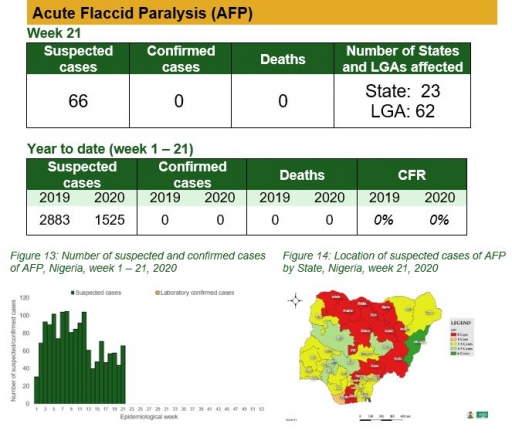
Acute Flaccid Paralysis (AFP)
Key points
• There were 66 suspected cases of AFP reported from 62 LGAs in 23 states. None was laboratory confirmed and no death was recorded
Coronavirus (COVID-19)
Actions
To date:
• National COVID-19 multi-partner Emergency Operations Centre (EOC) continues to coordinate response activities across states
• Shared strategies to improve sample collection with states
• Accredited and activated thirty (30) testing laboratories across Nigeria so far
• Continue to receive and quarantine returnees from other countries
• Printed and distributed the mandatory institutional quarantine guideline and NCDC CARE kit for the returnees and evacuees
• National Rapid Response Team continues to support affected states
• Conducted training on risk communication and virtual reporting training for media practitioners across the country
• Ongoing deployment of commodities to support affected states based on needs.
• Developed Home care interim guideline for COVID-19 patients which can be accessed via covid19.ncdc.gov.ng
Planned:
• Work with states to scale up sample collection and testing
• Continue mobilisation of resources
• Continue to provide guidance to states in line with national guidelines and global best practices
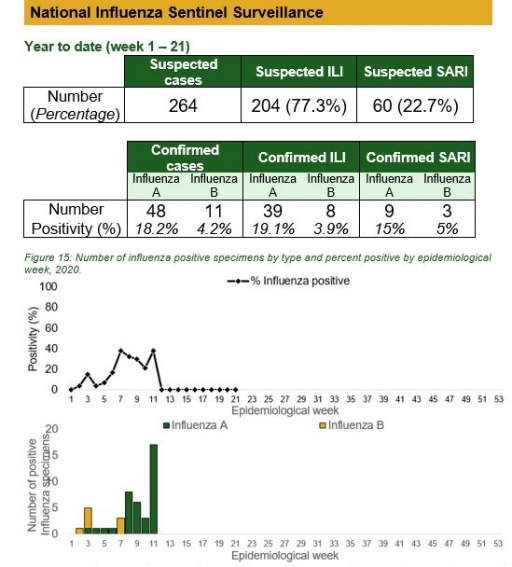
National Influenza Sentinel Surveillance
Key points
• The subtypes A seasonal H3, 2009A/H1N1 and A/not subtyped account for 0 (0.0%), 2 (9.5%) and 19 (90.5%) of the total influenza A positive sample respectively. The subtypes B VICTORIA, B Not subtyped and B Yamagata account for 0 (0.0%), 8 (100%) and 0 (0.0%) of the total influenza B positive samples respectively.
• The percentage influenza positive was highest in week 10 with 40%.
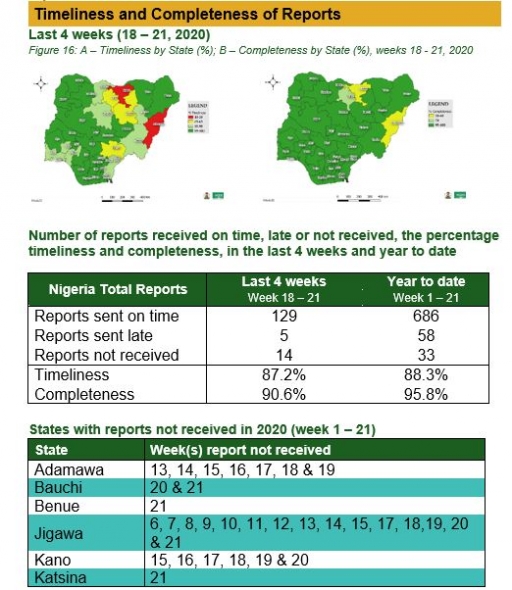
Timeliness and Completeness of Reports
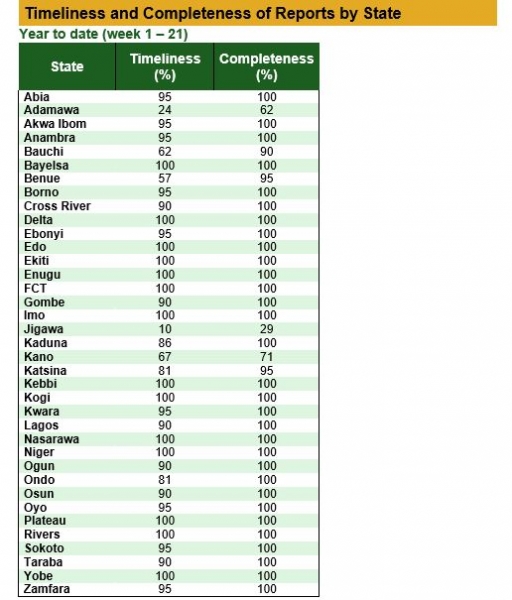
Timeliness and Completeness of Reports by State














 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 