Editoral
Fostering public understanding: NCDC and states’ roles in COVID-19 testing and management pathways in Nigeria
Posted: 19-05-2020 04:26:24 PM
The Nigeria Centre for Disease Control (NCDC) is an agency of the Federal Ministry of Health with the mandate to protect the health of Nigerians against deadly epidemic prone diseases (EPDs) and other public health threats. In line with this, the NCDC has been leading the public health response to the ongoing the coronavirus disease (COVID-19) outbreak in Nigeria. This is in addition to the concurrent response to other EPDs such as measles, cerebrospinal meningitis, monkeypox, cholera and viral haemorrhagic fevers such as Lassa fever etc.
Given the novelty of this virus and continuous evolving knowledge about it, we are continuously faced with questions on delineation of roles of the NCDC and states in the COVID-19 response particularly on diagnostics and case management pathways. Questions like Who leads the response at the state level? Who is responsible for setting up testing centre(s) in states? Whose responsibility is it to establish and accredit testing laboratories? How does NCDC collate and publish national results? Does NCDC build and manage quarantine and isolation centres? etc.
To address these public concerns, we highlight critical roles of the NCDC and states vis a vis the afore-mentioned pathways of response. These are:
1. The states lead COVID-19 response in their respective states while the NCDC’s rapid response team (RRT) continue to work closely with them by providing all necessary guidelines, technical and logistics support.
2. It is the responsibility of the state RRT to collect samples from all suspected cases that meet the case definition criteria. The NCDC on the other hand continues to provide guidelines and protocols on this.
3. Sample collection centres are owned by the states. However, the NCDC supports in some instances, the transportation of samples to the COVID-19 testing centres, especially for states without laboratories.
4. The NCDC provides guidance on how laboratory should be set up and operationalised. Currently, of the 26 accredited and activated laboratories by the NCDC, a total of 25 laboratories are being run by the states and Federal institutions where they are domiciled. Therefore, it is the responsibility of the accredited laboratories to test all samples in line with the laid down guidelines. The NCDC only owns the National Reference Laboratory, Gaduwa, Abuja and the Central Public Health Laboratory, Yaba, Lagos.
5. On a daily basis, NCDC collates and publishes all results as shared by the testing laboratories from reporting states. Currently, NCDC is working closely with states, using Surveillance Outbreak Response Management Analysis System (SORMAS) to provide real-time reporting of laboratory results and cases.
6. The NCDC does not build quarantine or treatment centres. This is the responsibility of the State Government or relevant teaching hospitals. However, the NCDC provides guidance on the construction of standard isolation centres and continues to provide national case management guidelines (including discharge protocol) as well as training of case managers, in line with the global best practices for COVID-19.
These are a few of the critical roles of the NCDC and states regarding diagnostics and case management, in the national response to COVID-19. We commend the leadership and efforts of states in the fight against the virus and urge for more efforts in the area of resource mobilisation, for all aspects of the response. The public are also encouraged to contact their state helplines for more information on state specific services such as the location of sample collection centres. The NCDC remains committed to supporting states to strengthen their health security.
Summary of Incidents
Notes
1. Information for this disease was retrieved from the Technical Working Group and Situation Reports
2. Case Fatality Rate (CFR) for this disease is reported for confirmed cases only
3. Information for this disease was retrieved from IDSR 002 data
4. CFR for this disease is reported for total cases i.e. suspected + confirmed
5. Information for sentinel influenza was retrieved from the laboratory
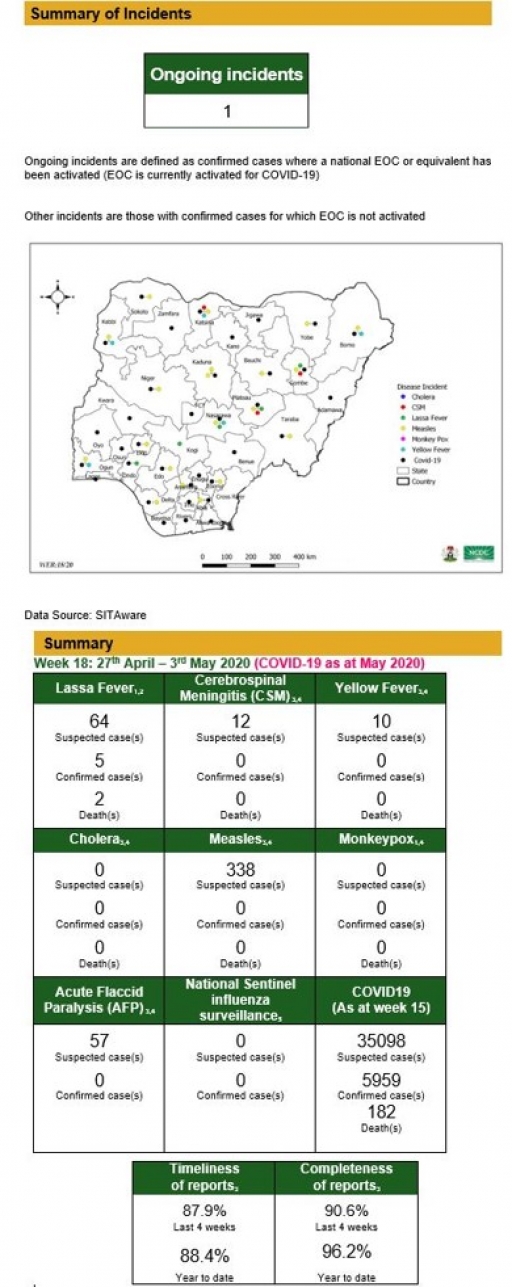
Lassa Fever
Key points
• There were 64 suspected cases, five confirmed cases and two deaths were recorded from 37 LGAs in 15 states
Actions
To date:
• National rapid response and surge teams have been deployed from NCDC to support response activities in states
• State Public Health Emergency Operations Centre activated in affected States
• Developed Incident Action Plan
• High level advocacy visit by the Honourable Minister of Health and DG of NCDC to Kano State
• Implementation of Lassa fever environmental response campaign in high burden states by the Federal Ministry of Environment
Planned:
• Resource mobilisation
• Pilot indigent patient treatment scheme through the basic healthcare provision funds
• Support states to develop and implement LF response sustainability plan
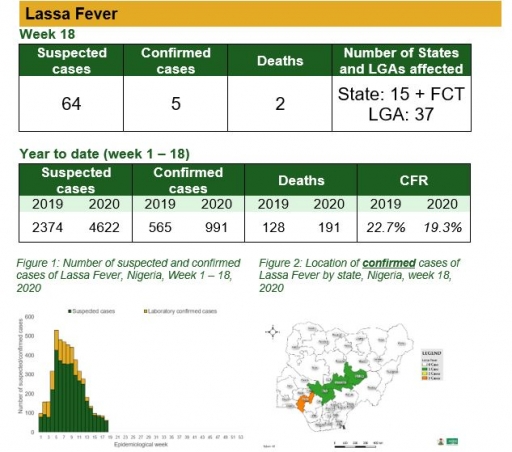
Cerebrospinal Meningitis (CSM)
Key points
There were 12 suspected cases of Cerebrospinal Meningitis (CSM) reported from six LGAs in four states (Gombe – 1, Kaduna – 1, Katsina – 9 & Plateau – 1). None was laboratory confirmed and no death was recorded
Actions
To date:
• National CSM TWG meets weekly to review reports from states and plan appropriately
• Enhanced surveillance in all states
Planned:
• Continue harmonisation of the national line list and SORMAS data
• Continue to ensure that states reporting cases send their line lists and collect CSM samples
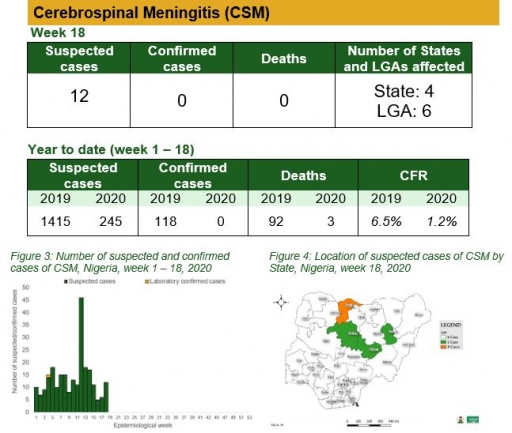
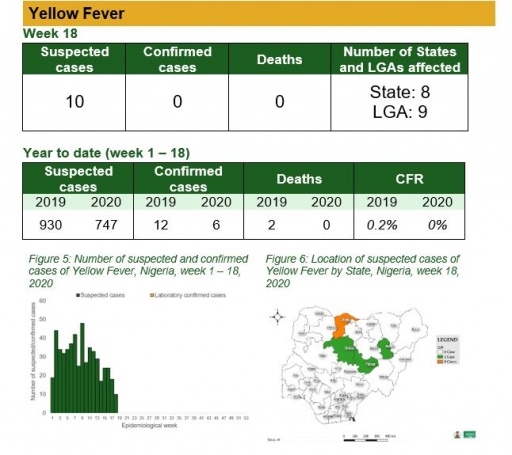
Yellow Fever
Key points
• There were 10 suspected cases of Yellow Fever (YF) reported from nine LGAs in eight states. None was laboratory confirmed and no death was recorded
Actions
To date:
• National multiagency YF Technical Working Group (TWG) is coordinating response activities
Planned:
• Surveillance and laboratory data harmonisation are ongoing
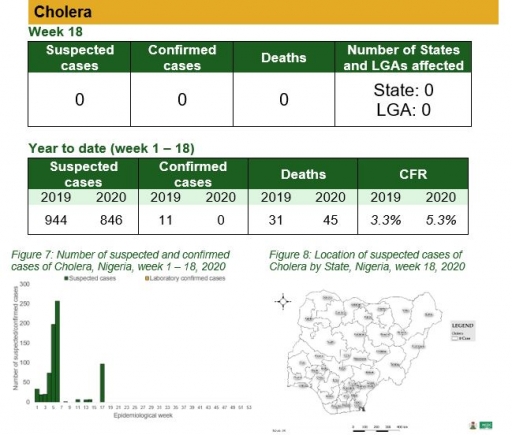
Cholera
Key points
• There was no case of cholera reported this week
Actions
To date
• National cholera multi-sectoral Technical Working Group (TWG) is monitoring all states and supporting already affected states
Planned:
• Continue follow up and monitoring of non-reporting states
• Continue harmonisation of the national line list and SORMAS data
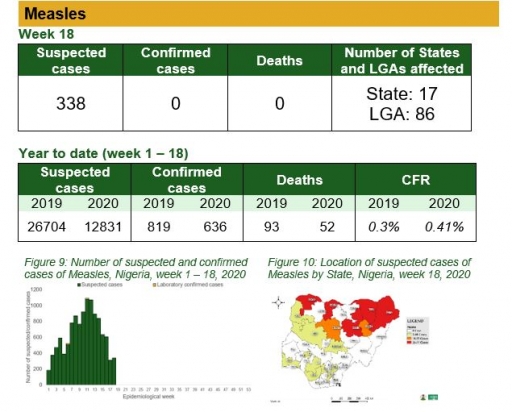
Measles
Key points
• There were 338 suspected cases of Measles reported from 86 LGAs in 17 states. None was laboratory confirmed and no death was recorded
Actions
To date
• National Measles Technical Working Group (TWG) is closely monitoring surveillance data and response activities across the country
Planned:
• Follow up with states to update and transmit line list
• Continue the review of measles surveillance data across the country
• Continue harmonisation of the national line list and SORMAS data
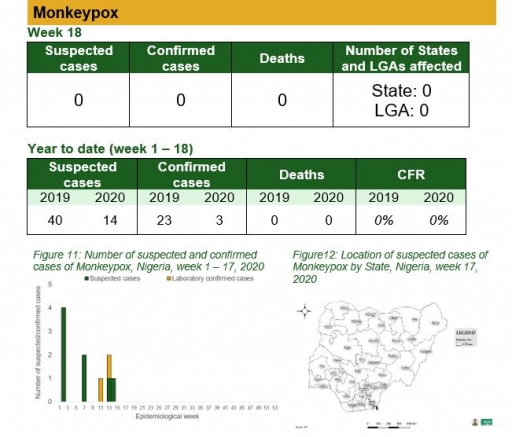
Monkeypox
Key points
• There was no case of monkeypox reported this week
Actions
To date
• National Monkeypox Technical Working Group (TWG) is monitoring activities in all states
Planned:
• Enhance surveillance for monkeypox in high burden states
• Continue harmonisation of the national line list and SORMAS data
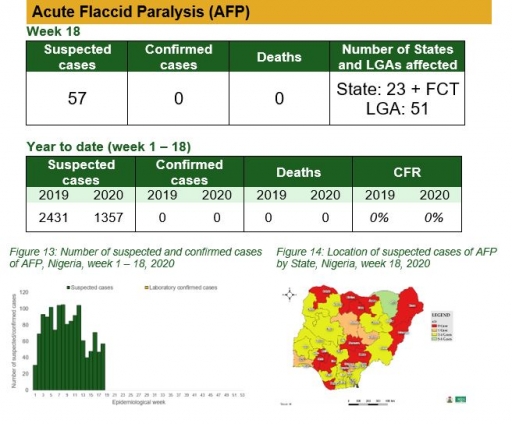
Acute Flaccid Paralysis (AFP)
Key points
• There were 57 suspected cases of AFP reported from 51 LGAs in 23 states and FCT. None was laboratory confirmed and no death was recorded
Coronavirus (COVID-19)
Actions
To date:
• National Emergency COVID-19 (LF) multi-partner, multi-sectoral Operations Centre (EOC) activated at level continues to coordinate response activities across states.
• Accredited and activated twenty-six (26) testing laboratories across Nigeria so far
• Trained 3rd batch Health Care Workers (HCWs) in Kano state
• Printed and distributed the mandatory institutional quarantine guideline and NCDC CARE kit for the returnees and evacuees
• National Rapid Response Team continues to support affected states.
• Developed Home care interim guideline for COVID-19 patients
Planned:
• Continue mobilisation of resources
• Operationalisation of next strategic directions following the COVID-19 National Outbreak Response Mid-action review meeting
• Continue to establish surge capacities across all response pillars
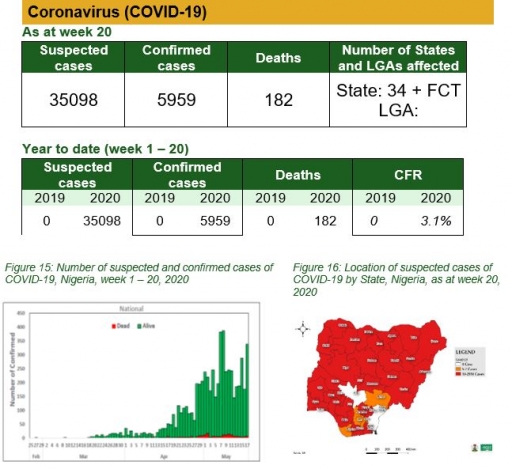
National Influenza Sentinel Surveillance
Key points
• The subtypes A seasonal H3, 2009A/H1N1 and A/not subtyped account for 0 (0.0%), 2 (9.5%) and 19 (90.5%) of the total influenza A positive samples respectively. The subtypes B VICTORIA, B Not subtyped and B Yamagata account for 0 (0.0%), 8 (100%) and 0 (0.0%) of the total influenza B positive samples respectively.
• The percentage influenza positive was highest in week 10 with 40%.
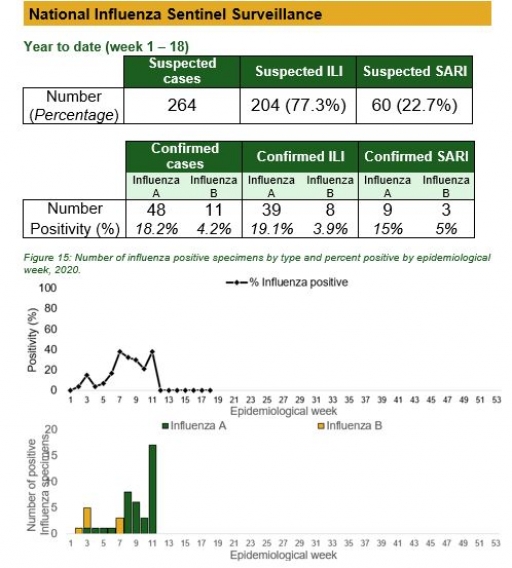
Timeliness and Completeness of Reports
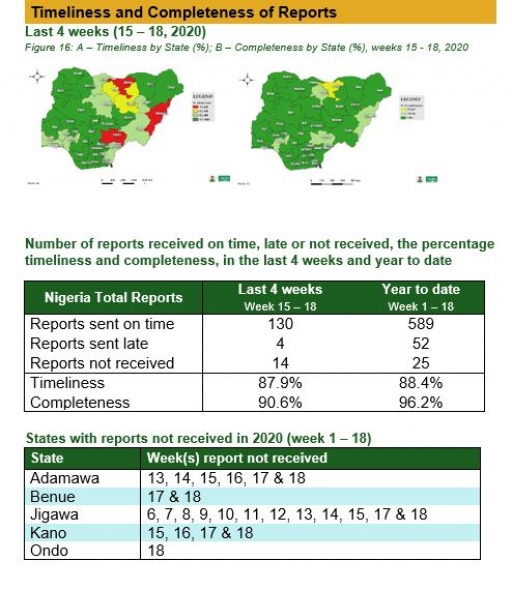
Timeliness and Completeness of Reports by States













 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 