Since the first case of coronavirus disease (COVID-19) was reported in Nigeria in February 2020, the Nigeria Centre for Disease Control (NCDC) has continued to lead public health response activities. With the evolution of the pandemic and scientific guidance for response activities, NCDC has continued to introduce diverse response measures. One critical priority for the response, is scale up of access to testing.
As part of measures to scale up access to testing, NCDC has worked with States and partners to establish walk-in sites for sample collection, increase communications around testing and activate at least one testing laboratory in every state and the Federal Capital Territory (FCT). Timely access to testing is critical for early diagnosis, effective case management and prompt tracing of likely contacts thereby limiting spread of the virus.
A major challenge in the COVID-19 response is the long turnaround time for testing, using the molecular polymerase chain reaction (PCR) method. The World Health Organisation has recently granted emergency use authorisation for Rapid Diagnostic Test (Ag-RDTs) kits, produced by Abott and SD-Biosensor.
In line with the above, NCDC carried out a national validation of these test kits and has now approved its inclusion in the country’s COVID-19 testing strategy. The use of these approved Ag-RDTs is limited to congregate settings such as camps, workplace, health facilities to triage patients etc. Given the limitations of these Ag-RDTs, molecular PCR remains the gold standard for testing.
The NCDC has begun a pilot use of the approved Ag-RDTs in five selected health facilities in FCT, Nigeria. The overall goal is to assess the cost effectiveness of implementing antigen detecting SARS-CoV-2 rapid diagnostic tests and use lessons learnt to scale-up to other health facilities across Nigeria. The pilot involved training of healthcare workers on detection, sample collection, packaging, transportation, testing and data collection.
The proper use of Ag-RDTs is expected to improve the national testing capacity by supporting the existing network of COVID-testing laboratories. We urge states and other public and private institutions to leverage on this plan, by ensuring appropriate use in the right settings. More information on the use of Ag-RDTs can be found here..
Summary of Incidents
Notes
1. Information for this disease was retrieved from the Technical Working Group and Situation Reports
2. Case Fatality Rate (CFR) for this disease is reported for confirmed cases only
3. Information for this disease was retrieved from IDSR 002 data
4. CFR for this disease is reported for total cases i.e. suspected + confirmed
5. Information for sentinel influenza was retrieved from the laboratory
Lassa Fever
Key points
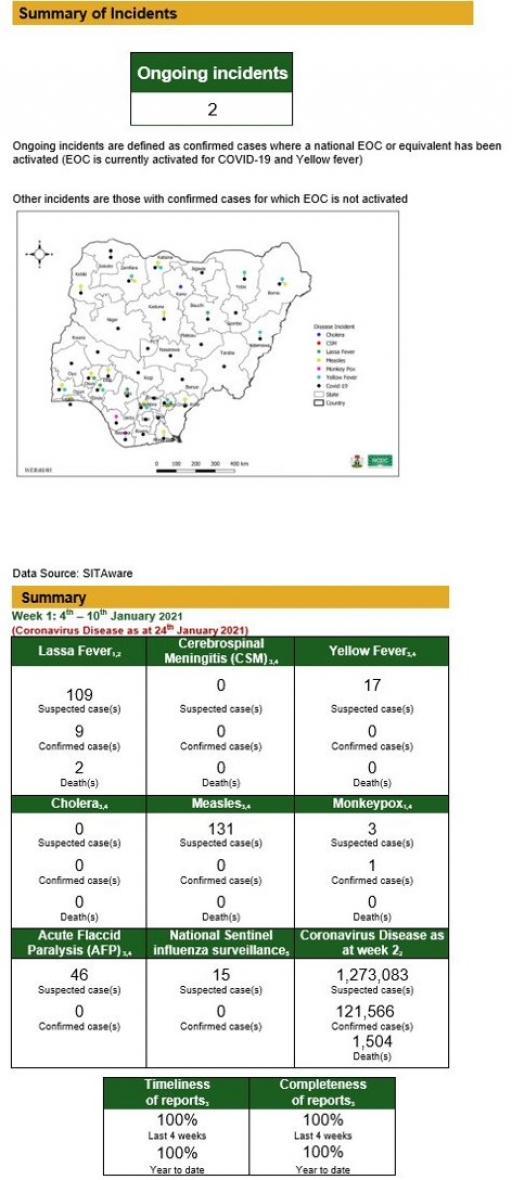
• There were 109 suspected cases, nine were laboratory confirmed and two deaths were recorded from four states
Actions
To date:
• The National multisectoral Lassa fever Emergency Operations Centre (EOC) was activated to coordinate response activities across states
• State Public Health Emergency Operations Centre activated in affected states
Planned:
• Continue mobilisation of resources
• Finalise Lassa fever five-year strategic plan
Cerebrospinal Meningitis (CSM)
Key points
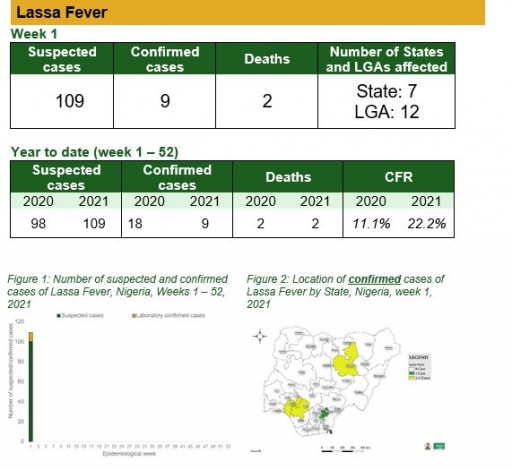
• There were no suspected cases of Cerebrospinal Meningitis (CSM) reported this week
Actions
To date:
• National CSM TWG meets weekly to review reports from states and plan appropriately
• Enhanced surveillance in all states
Planned:
• Continue harmonisation of the national line list and SORMAS data
• Continue to ensure that states reporting cases send their line lists and collect CSM samples
Yellow Fever
Key points
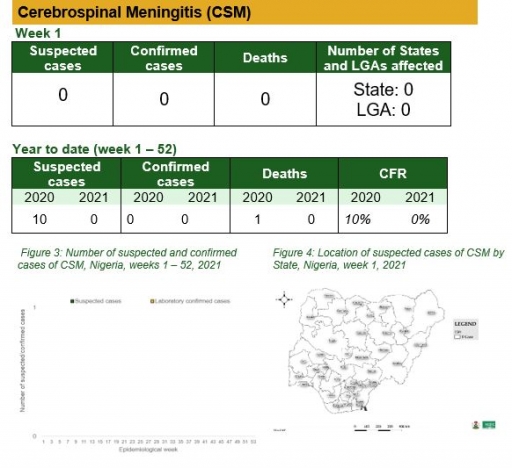
• There were 17 suspected cases of Yellow Fever (YF) reported from 14 LGAs in 10 states. None was laboratory confirmed and no death was recorded
Actions
To date:
• National YF multi-partner Emergency Operations Centre (EOC) continues to coordinate response activities across States
• Daily monitoring and analysis oof surveillance data across the country to guide response activities
• Concluded Accelerated Preventive Mass Vaccination (PMVC) Campaign in Enugu, Delta and PMVC in Bauchi State
Planned:
• Continue implementation of the Incident Action Plan (IAP) and mobilise required resources for the outbreak response
• Continue to support affected states across all pillars of response
• Continue harmonisation of surveillance and laboratory data ongoing
Cholera
Key points
• There were no cases of Cholera reported this week
Actions
To date
• National Cholera Multi-Sectoral Technical Working Group (TWG) is monitoring all states and supporting affected states
Planned:
• Continue follow up and monitoring of non-reporting states
• Continue harmonisation of the national line list and SORMAS data

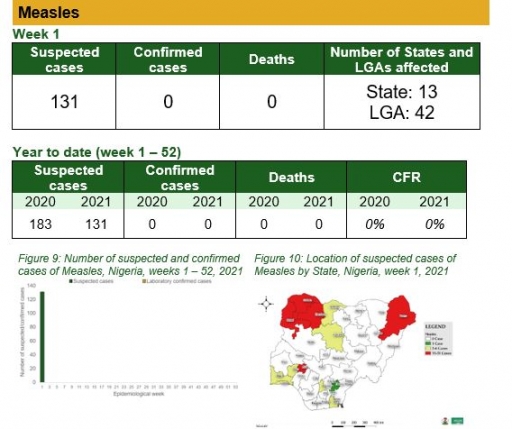
Measles
Key points
• There were 131 suspected cases of Measles reported from 42 LGAs in 13 States. There was no laboratory confirmed case and no death was recorded
Actions
To date
• National Measles TWG is closely monitoring measles surveillance data and providing feedback to relevant agencies and development partners
• Weekly surveillance and laboratory data harmonisation ongoing
Planned:
• Intensify follow up with states to update and transmit line list
• Continue monthly measles surveillance data review
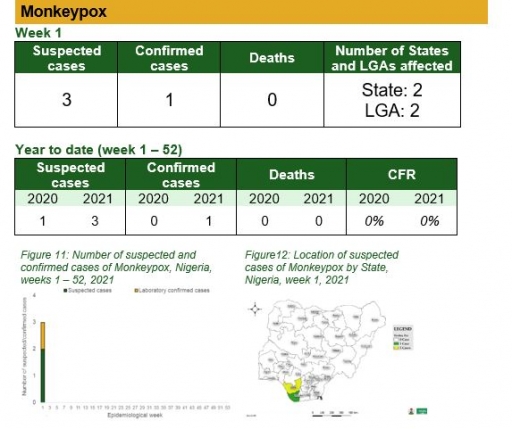
Monkeypox
Key points
• There were three suspected cases of Monkeypox reported from two LGAs in two States (Bayelsa – 1 & Delta – 2)
•
Actions
To date
• National Monkeypox Technical Working Group (TWG) is monitoring activities in all states
Planned:
• Enhance surveillance for monkeypox in high burden states
• Continue harmonisation of the national line list and SORMAS data
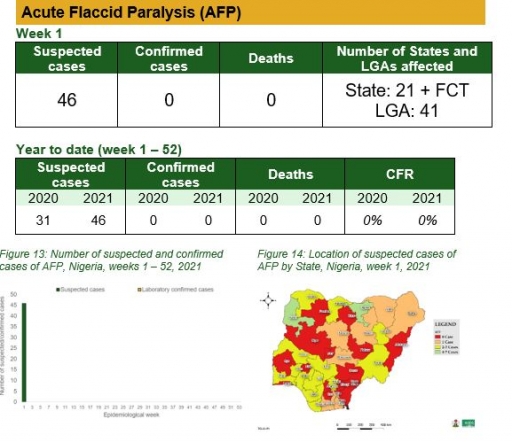
Acute Flaccid Paralysis (AFP)
Key points
• There were 46 suspected cases of AFP reported from LGAs in 21
• States. None was laboratory confirmed and no death was recorded
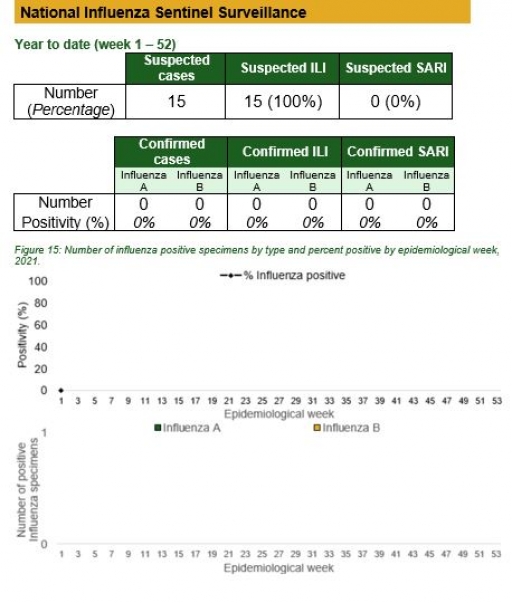
National Influenza Sentinel Surveillance
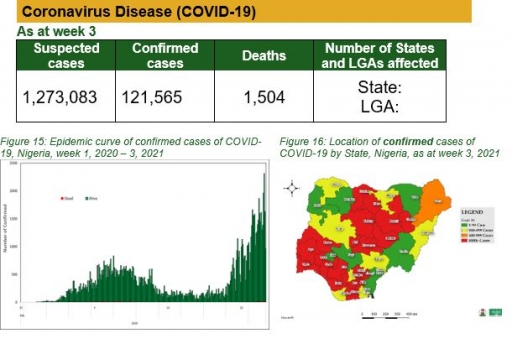
Coronavirus Disease (COVID-19)
Actions
To date:
• National COVID-19 multi-partner Emergency Operations Centre (EOC) continues to coordinate response activities across States
• Published and disseminated the updated COVID-19 case definition to stakeholders
• Intensified follow up with States to collect data on cases and contacts
• Trained healthcare workers on IPC in Nasarawa State
• Supported National Primary Health Care Development Agency (NPHCDA) with sensitization of religious leaders and civil society organisations (CSOs) on planned vaccination
Planned:
• Deploy additional Rapid Response Teams to support States
• Finalise Local Government Area (LGA)/State transmission categorisation
• Support supervisory visits to private laboratories in FCT
Timeliness and Completeness of Reports
Timeliness and Completeness of Reports by State













 Toll Free Number: 6232
Toll Free Number: 6232 Whatsapp: +234 708 711 0839
Whatsapp: +234 708 711 0839 SMS Number: +234 809 955 5577
SMS Number: +234 809 955 5577 